All Photos(1)
About This Item
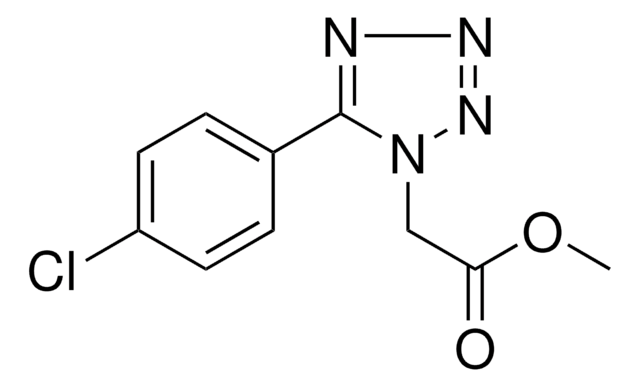
Empirical Formula (Hill Notation):
C7H5ClN4
CAS Number:
Molecular Weight:
180.59
Beilstein:
139070
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
120-123 °C (lit.)
solubility
dichloromethane: soluble 25 mg/mL, clear, colorless
SMILES string
Clc1nnnn1-c2ccccc2
InChI
1S/C7H5ClN4/c8-7-9-10-11-12(7)6-4-2-1-3-5-6/h1-5H
InChI key
DHELIGKVOGTMGF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Chloro-1-phenyl-1H-tetrazole reacts with 2-hydroxyacetophenone to yield 1-phenyl-1H-tetrazol-5-oxy linked compound.
Application
5-Chloro-1-phenyl-1H-tetrazole was used as the reagent during the synthesis of new glycosyl donor possessing an anomeric O-(1-phenyltetrazol-5-yl) group.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Palme et al.
Bioorganic & medicinal chemistry, 2(11), 1169-1177 (1994-11-01)
A new glycosyl donor possessing an anomeric O-(1-phenyltetrazol-5-yl) group is prepared from 2,3,4,6-tetra-O-benzyl-D-glucose (2) and commercially available 5-chloro-1-phenyl-1H-tetrazole (1). The synthesis of glycosides derived from the donor and a few primary and secondary alcohols is reported.
Scott P Webster et al.
Bioorganic & medicinal chemistry letters, 20(11), 3265-3271 (2010-05-11)
Inhibitors of 11beta-hydroxysteroid dehydrogenase (11beta-HSD1) show promise as drugs to treat metabolic disease and CNS disorders such as cognitive impairment. A series of 1,5-substituted 1H-tetrazole 11beta-HSD1 inhibitors has been discovered and chemically modified. Compounds are selective for 11beta-HSD1 over 11beta-HSD2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service