C3023
CHAPS hydrate
≥98% (HPLC)
Synonym(s):
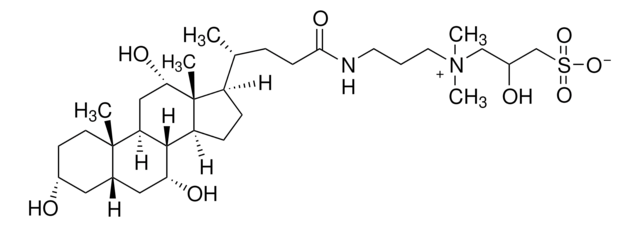
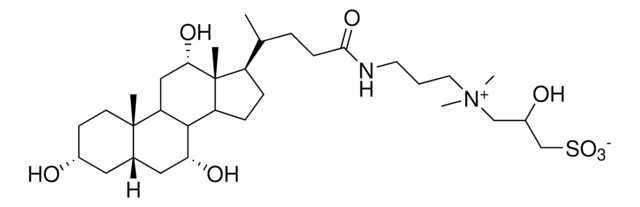
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate, CHAPS
About This Item
Recommended Products
biological source
bovine bile
Quality Level
grade
HPLC grade
description
zwitterionic
Assay
≥98% (HPLC)
form
powder
mol wt
614.88 g/mol (anhydrous basis)
storage condition
dry at room temperature (tightly closed)
technique(s)
dialysis: suitable
electrophoresis: suitable
impurities
<0.04% DMF
CMC
8 mM
mp
157 °C (dec.) (lit.)
transition temp
cloud point >100 °C
solubility
water: 50 mg/mL
shipped in
ambient
storage temp.
2-30°C
SMILES string
O.C[C@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI
1S/C32H58N2O7S.H2O/c1-21(8-11-29(38)33-14-6-15-34(4,5)16-7-17-42(39,40)41)24-9-10-25-30-26(20-28(37)32(24,25)3)31(2)13-12-23(35)18-22(31)19-27(30)36;/h21-28,30,35-37H,6-20H2,1-5H3,(H-,33,38,39,40,41);1H2/t21-,22+,23-,24-,25+,26+,27-,28+,30+,31+,32-;/m1./s1
InChI key
SJCUTFKCLFLIFE-JWTJKVBLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Widely utilized as a buffer in various research applications, CHAPS hydrate acts as an amphiphilic surfactant, proving effective in solubilizing plasma membrane proteins and diluting assay components. Notably, its easy removal through dialysis or gel filtration adds to its appeal, making it a preferred choice for molecular mass determination by fluorometry. This feature underscores CHAPS hydrate′s adaptability in diverse experimental setups. However, caution is advised in assays involving polyvalent metals or metal ions sensitive to nonspecific chelating agents. Beyond its conventional use, CHAPS hydrate finds application as a polymer matrix in binding assays, effectively removing metal ions from protein solutions.
Application
Features and Benefits
- Suitable for Biochemical and Cell Biology research
- Can be used in Dialysis and Electrophoresis
- High purity product for research applications
Other Notes
comparable product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This page shows how to solubilize membrane proteins with products from Cytiva.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service