All Photos(1)
About This Item
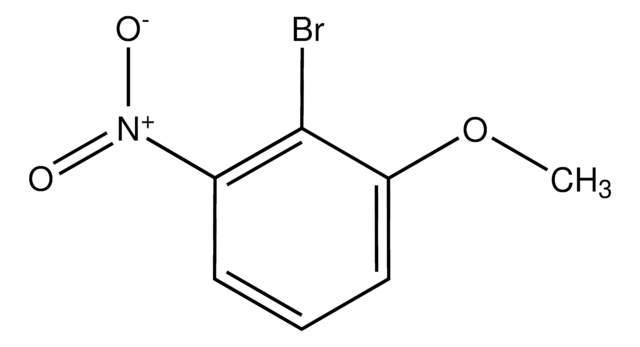
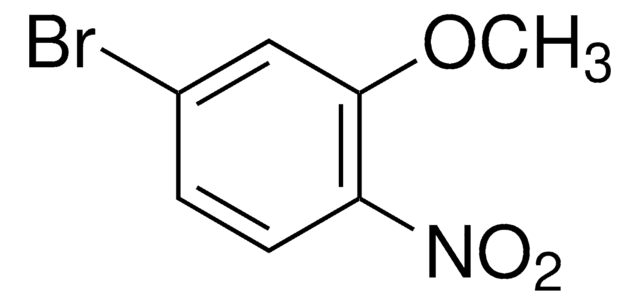
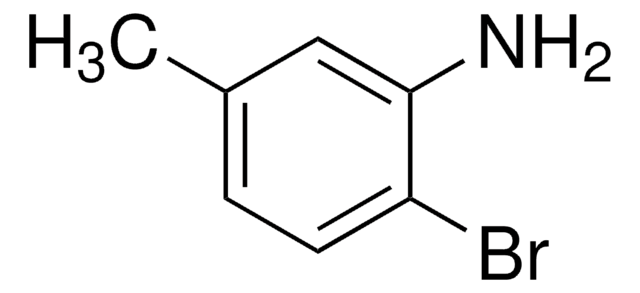
Linear Formula:
BrC6H3(NO2)OCH3
CAS Number:
Molecular Weight:
232.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
153-154 °C/13 mmHg (lit.)
mp
32-34 °C (lit.)
SMILES string
COc1ccc(Br)c(c1)[N+]([O-])=O
InChI
1S/C7H6BrNO3/c1-12-5-2-3-6(8)7(4-5)9(10)11/h2-4H,1H3
InChI key
KCOBIBRGPCFIGF-UHFFFAOYSA-N
General description
FT-IR and FT-Raman spectra of 4-bromo-3-nitroanisole have been studied.
Application
4-Bromo-3-nitroanisole was used in the synthesis of:
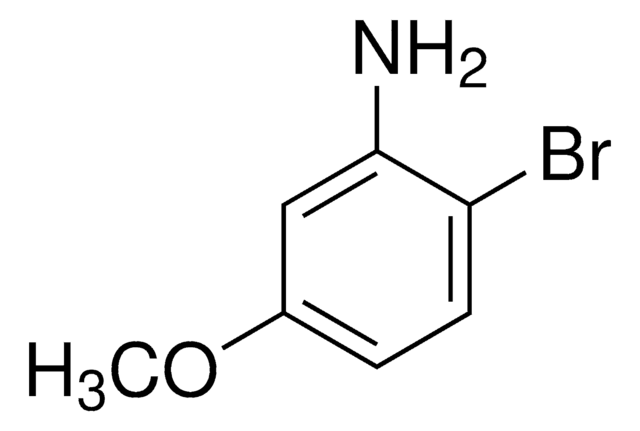
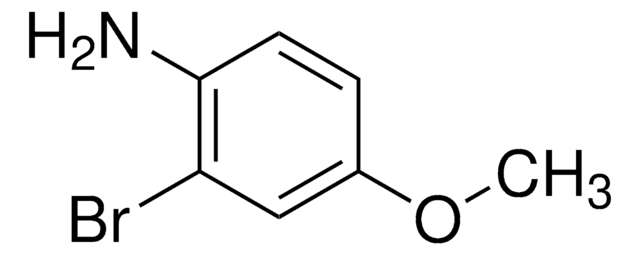
- 4-bromo-5-methoxyaniline
- 2-(4-methoxy-2-nitrophenylsulfanyl)benzoic acid
- 4-(1-diethylamino-4-pentylamino)-3-nitrochlorobenzene
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 106, 284-298 (2013-02-19)
The FT-IR and FT-Raman spectra of 4-bromo-3-nitroanisole (BNA) molecule have been recorded in the region 4000-400 cm(-1) and 3500-100 cm(-1), respectively. Optimized geometrical structure, harmonic vibrational frequencies, intensities, reduced mass, force constants and depolarization ratio have been computed by ab
Synthesis of soluble polyarylenes containing alternating 4, 4'-(1, 1'-binaphthyl) and 4, 4'-(3, 3'-diphenyl) biphenyl structural units.
Percec V, et al.
Polymer Bull., 29(3-4), 271-276 (1992)
Some derivatives of 3-amino-4-(1-diethylamino-4-pentylamino)-chlorobenzene.
R L McKEE et al.
Journal of the American Chemical Society, 68(10), 1904-1904 (1946-10-01)
A A Wilson et al.
Journal of medicinal chemistry, 43(16), 3103-3110 (2000-08-24)
A series of four 2-(phenylthio)araalkylamines have been radiolabeled with (11)C and evaluated as potential radiotracers for imaging the serotonin transporter (SERT) by positron emission tomography (PET). All four candidates display high affinity for SERT and low affinity for the dopamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service