SML2521
BRD6929
≥98% (HPLC)
Synonym(s):
4-(Acetylamino)-N-[2-amino-5-(2-thienyl)phenyl]benzamide, BRD6929, Compound 60, TPB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H17N3O2S
CAS Number:
Molecular Weight:
351.42
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
SMILES string
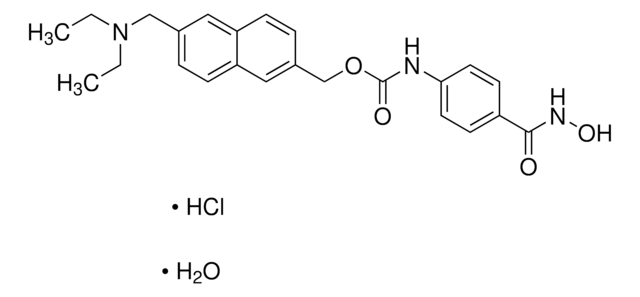
CC(NC1=CC=C(C(NC2=C(N)C=CC(C3=CC=CS3)=C2)=O)C=C1)=O
Biochem/physiol Actions
TPB is a potent and selective inhibitor of HDAC1 and HDAC2. TPB potentiates gnidimacrin activation of latent HIV-1 in cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Li Huang et al.
ACS medicinal chemistry letters, 9(3), 268-273 (2018-03-16)
We have previously reported gnidimacrin (GM), a protein kinase C (PKC) agonist, significantly reduces the frequency of HIV-1 latently infected cells in peripheral blood mononuclear cells (PBMCs) from patients undergoing successful antiretroviral therapy at low picomolar concentrations ex vivo, which
Andrew J Wilson et al.
Cancer biology & therapy, 12(6), 484-493 (2011-07-09)
High grade epithelial ovarian cancers are relatively sensitive to DNA damaging platinum-based chemotherapy, suggesting that the dependencies of ovarian tumors on DNA damage response pathways can be harnessed for therapeutic purposes. Our goal was to determine if the DNA damage
Joey L Methot et al.
Bioorganic & medicinal chemistry letters, 18(3), 973-978 (2008-01-10)
We report herein the initial exploration of novel selective HDAC1/HDAC2 inhibitors (SHI-1:2). Optimized SHI-1:2 structures exhibit enhanced intrinsic activity against HDAC1 and HDAC2, and are greater than 100-fold selective versus other HDACs, including HDAC3. Based on the SAR of these
F F Wagner et al.
Chemical science, 6(1), 804-815 (2015-02-03)
Aiming towards the development of novel nootropic therapeutics to address the cognitive impairment common to a range of brain disorders, we set out to develop highly selective small molecule inhibitors of HDAC2, a chromatin modifying histone deacetylase implicated in memory
Stefan Kubicek et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(14), 5364-5369 (2012-03-22)
Under the instruction of cell-fate-determining, DNA-binding transcription factors, chromatin-modifying enzymes mediate and maintain cell states throughout development in multicellular organisms. Currently, small molecules modulating the activity of several classes of chromatin-modifying enzymes are available, including clinically approved histone deacetylase (HDAC)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service