All Photos(2)
About This Item
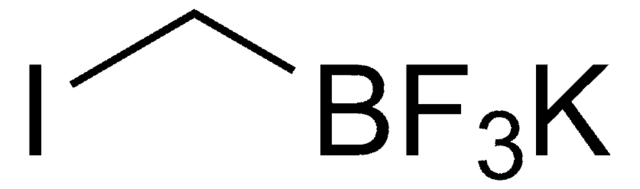
Empirical Formula (Hill Notation):
C2BF8K
CAS Number:
Molecular Weight:
225.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
252-257 °C
storage temp.
2-8°C
SMILES string
F[B-](F)(F)C(F)(F)C(F)(F)F.[K+]
InChI
1S/C2BF8.K/c4-1(5,2(6,7)8)3(9,10)11;/q-1;+1
InChI key
PSJPJAFBTMLFFX-UHFFFAOYSA-N
Related Categories
Application
Organotrifluoroborate involved in:
Organotrifluoroborates as versatile and stable boronic acid surrogates
- Suzuki Miyaura cross-coupling reactions, and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jenny M Baxter Vu et al.
Organic letters, 13(15), 4056-4059 (2011-07-14)
A new two-step synthesis of highly substituted pyrrolidines has been developed. Chiral silane Lewis acid promoted enantioselective Mannich reactions of silyl ketene imines with acylhydrazones may be used to access bishomoallylic benzoic hydrazides that in turn may be cyclized to
R. Grisorio;
Journal of Polymer Science: Part A, General Papers, 49, 842-847 (2011)
Gary A Molander et al.
Journal of the American Chemical Society, 130(47), 15792-15793 (2008-11-05)
A method was developed for the hydroboration of alkenyl-containing organotrifluoroborates to generate dibora intermediates. The reactivity differences between organotrifluoroborates and trialkylboranes facilitated the cross-coupling of the borane moiety of these intermediates in a highly chemoselective fashion with aryl halides, leaving
S. Achelle;
Journal of Polymer Science: Part A, General Papers, 48, 2659-2665 (2010)
Emilio Alacid et al.
The Journal of organic chemistry, 74(6), 2321-2327 (2009-02-17)
Potassium vinyl and alkenyltrifluoroborates are cross-coupled with aryl and heteroaryl bromides using 1 mol % Pd loading of 4-hydroxyacetophenone oxime derived palladacycle or Pd(OAc)2 as precatalysts, K2CO3 as base, and TBAB as additive and water reflux under conventional or microwave
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Phenyl[3-(trifluoromethyl)phenyl]iodonium triflate ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/424/062/057593f4-e032-4d3e-bed6-6be37c1ae76d/640/057593f4-e032-4d3e-bed6-6be37c1ae76d.png)


