Receptor Agonists and Antagonists
To be successful in drug discovery and pharmaceutical research, you must have a good grasp of the different kinds of receptor agonists and receptor antagonists. Learn the various types of receptor agonists and antagonists below.
What Are Receptor Agonists?
A receptor agonist is a molecule that can interact with the receptor and elicit a biological response. In most cases, it has a positive modulatory effect. Agonists display both affinity and intrinsic activity. They are mimetics of the natural ligand and produce biological effects that are on par with natural ligands. They bind to the same binding site and generate a complete response (full agonist), partial response (partial agonist), or inverse response (inverse agonist), as shown in Figure 1.
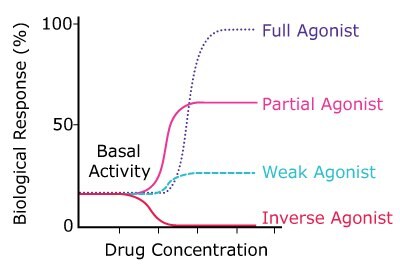
Figure 1. Action of a full, partial, and inverse agonist.
Full Agonists vs Partial Agonists
Full agonists display high efficacy for activating receptor function. For example, isoproterenol (Product No.420355) mimics the action of adrenaline on β-adrenergic receptors with a similar magnitude of action.
Partial agonists do bind to a receptor but only elicit a partial response. For example, buprenorphine does not exhibit full efficacy and is used for the treatment of opioid dependency. Indeed, partial agonists often do not have reduced affinity for the receptor binding site and can easily act to competitively block the responses produced by full agonists. The molecular mechanism for the partial agonist effect is not exactly clear, but likely the partial agonist causes a different receptor confirmation than a full agonist, which results in a diminished pharmacological response.
In some cases, a co-agonist may be required to produce the desired effect. For example, in NMDA (n-methyl-D-aspartic acid) receptor activation, glycine is required as a co-agonist in addition to glutamate.
Inverse Agonists
Inverse agonists are ligands that bind to the same receptor binding site but produce an effect that is opposite to that of the full or partial native ligand. They reduce the pharmacological response below the basal level, or resting level of activity, despite binding to the same receptor site as their full agonist counterpart. Although not common in natural ligand signaling pathways, an inverse agonist effect is sometimes the goal of neural and neuroendocrine drug design to generate either a positive or negative physiological response. An example of an inverse agonist is prazosin (Product No. P7791), which acts inversely on α1-adrenergic receptors.
What Are Receptor Antagonists?
Receptor antagonists are compounds that bind to a receptor and prevent the effect of an agonist. They are also known as receptor blockers. An antagonist can bind to the receptor, but it does not initiate a change in cellular function. Hence, they have the affinity, but no biological activity.
Antagonists can act by binding either to the same site as agonists or other unique sites, but they lack any intrinsic activity. In other words, their binding does not result in a biological response. Antagonist receptor binding can have similar kinetics to agonist binding, ranging from weak avidity to irreversible binding. However, most potent pharmacologically active receptor-binding molecules are reversible, display moderate avidity, are competitive with natural ligands, and are antagonistic in nature.
Competitive (Reversible) Antagonists
Competitive antagonists (also known as reversible antagonists) are molecules that compete with an agonist for the same binding site. Their blocking effect can be reversed by increasing the amount of agonist.
When high concentrations of an agonist result in the occupation of all receptor sites, higher amounts of the antagonist will be required to obtain the same degree of occupancy. In functional assays using competitive antagonists, parallel rightward shifts of agonist dose-response curves are observed but with no alteration of the maximal response (Figure 2). The level of activity of the receptor is determined by the relative affinity of each molecule for the site and their relative concentrations.

Figure 2.Effect of a competitive (reversible) antagonist.
Noncompetitive (Irreversible) Antagonists
Noncompetitive antagonists (also known as irreversible antagonists) are reactive compounds that covalently bind to the receptor at a distinctly separate binding site from the agonist, but in close proximity to the active site or an allosteric site. They do not compete with the agonist for binding.
Their binding is often irreversible, and they prevent receptors from adopting any conformational change required for their activation. Their binding affinity is high and, at a given time, a few, most, or all receptors can be occupied by the antagonist. Their duration of action is independent of the rate of their elimination and is more dependent on the turnover rate of the receptor molecules. Once the receptor is occupied, there is no need for any free antagonist to be present to block agonist responses.
Noncompetitive antagonists reduce the magnitude of the maximum response, which can be attained by any given amount of agonist, and their effects cannot be canceled. For this reason, these molecules are also known as nonsurmountable antagonists. In functional assays with noncompetitive antagonists, a depression of the maximal response of agonist dose-response curves is produced (Figure 3). An example of a noncompetitive antagonist is (+)-MK 801 Maleate, which irreversibly blocks the NMDA glutamate receptor.

Figure 3. Effect of a noncompetitive (irreversible) antagonist.
Search agonists and antagonists for your receptor or pathway of interest with our user-friendly bioactive small molecules search tool.
For Research Use Only. Not For Use In Diagnostic Procedures.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?