Piroxicam Capsules-Assay and Organic Impurities Following United States Pharmacopoeia Pending Forum Method
Dr Sanjay Poman,
Mumbai Application Laboratory
India
Introduction
Piroxicam (Figure 1) is an oxicam NSAID (non-steroidal anti-inflammatory drug) and is used to relieve the symptoms of painful inflammatory conditions like arthritis.1
A simple, precise, and sensitive reverse-phase high-performance liquid chromatography (RP-HPLC) gradient method (Table 1) using an Ascentis® Express C18 column was adopted for the assay and organic impurities analysis of Piroxicam capsules as part of method modernization from the USP-NF.2 The method was assessed in relation to USP general chapters <621>, <1225>, and <1226>.
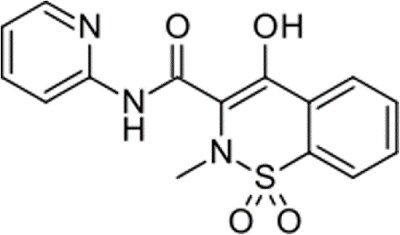
Figure 1.Chemical structure of Piroxicam
Piroxicam Capsules Assay And System Suitability Test
Experimental |
|---|
Results
Assay
Figure 2.Chromatographic blank run.
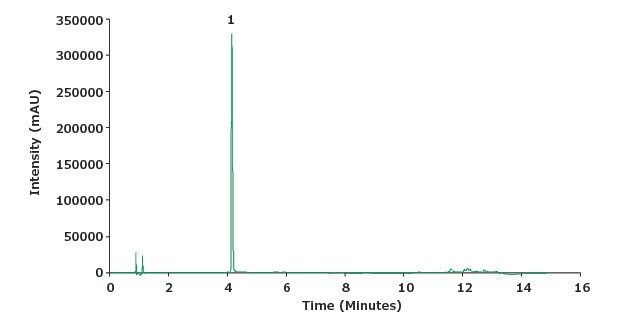
Figure 3.Chromatogram of a piroxicam standard solution (0.1 mg/mL).
Chromatographic Data for Standard Solution |
|---|
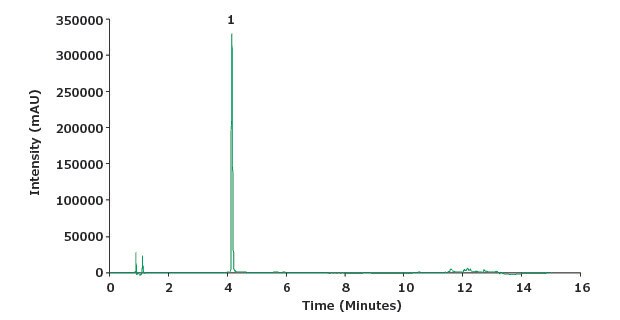
Figure 4.Chromatogram of piroxicam sample solution (0.1 mg/mL)
Chromatographic Data for Sample Solution |
|---|
System Suitability Requirements |
|---|
Organic Impurities Analysis of Piroxicam Capsules
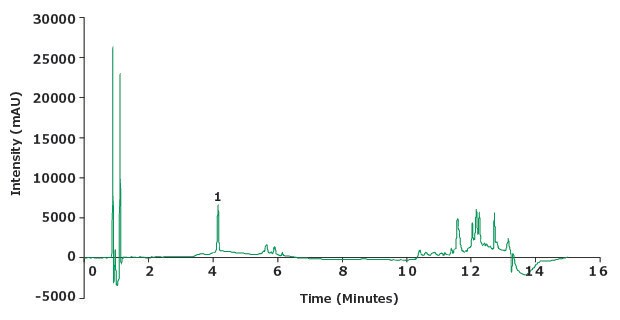
Figure 5.Chromatogram of the piroxicam sensitivity solution (0.5 µg/mL).
Chromatographic Data - Sensitivity Solution |
|---|
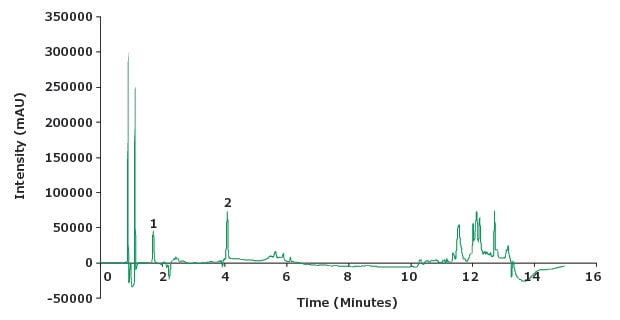
Figure 6.Chromatogram standard solution (2 µg/mL piroxicam and impurity A at 10 µg/mL).
Chromatographic Data – System Suitability Solution |
|---|

Figure 7.Chromatographic separation of standard solution organic impurities with 1 mg/mL piroxicam and impurity B at 10 µg/mL.
Chromatographic Data - Standard Solution Organic Impurities |
|---|
System Suitability Requirements* |
|---|
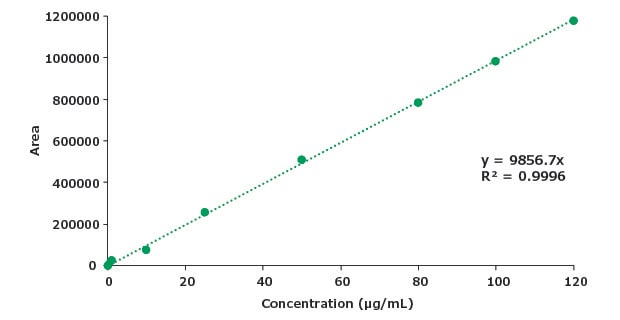
Figure 8.Calibration curve for piroxicam.
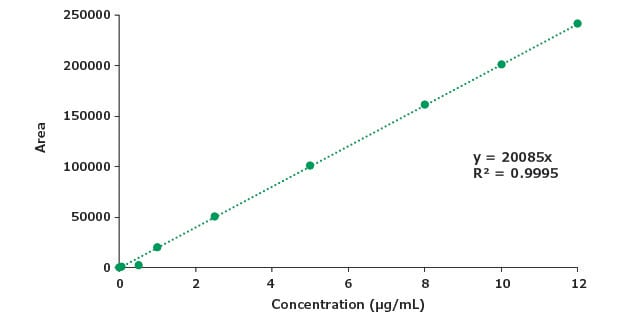
Figure 9.Calibration curve for impurity A.
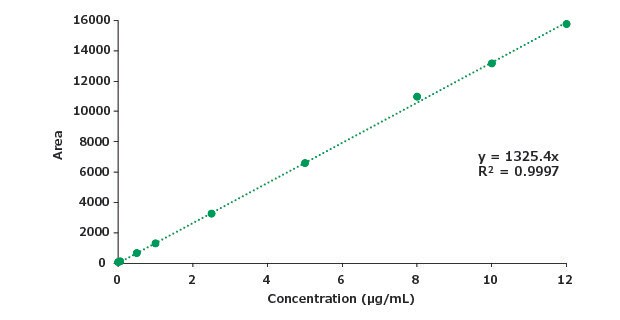
Figure 10.Calibration curve for impurity B.
Conclusion
A sensitive, stability indicating gradient reversed-phase HPLC method has been developed for the quantitative estimation of piroxicam (assay) and organic impurities in a dosage form. System suitability criteria mentioned in the USP monograph for reference standard as well as the test solution of piroxicam dosage form were found to be in compliance with % RSD and tailing factor parameters for the assay. The developed method was found to be linear for piroxicam up to 120 µg/mL with LOD of 4.2 µg/mL and LOQ of 12.9 µg/mL. For the organic impurity A linearity was assessed up to 12 µg/mL with LOD and LOQ at 0.4 and 1.3 µg/mL respectively, for impurity B also up to 12 µg/mL with LOD and LOQ at 0.37 and 1.1 µg/mL respectively. The relative retention times for impurities A and B are in agreement with the RRT values mentioned in the USP monograph. %RSD for piroxicam and piroxicam related compound A standard solution was not determined. The method can be applied for related substances study of piroxicam dosage form using the Ascentis® Express C18 HPLC column.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?