Determination of Vitamins B and Preservatives by HPLC-UV
Dr. Ajay Kaparwan
R&D, APAC, Jigani, India
Introduction
A multivitamin is a preparation intended to serve as a dietary supplement with nutritional elements like vitamins and minerals. Such preparations are available in the form of tablets, capsules, pastilles, powders, liquids or injectable formulations. Other than injectable formulations which are typically administered under medical supervision, multivitamins are recognized by the Codex Alimentarius Commission (the United Nations' authority on food standards) as a category of food.1
This study details the separation and quantification of water-soluble B Vitamins, methyl and propyl parabens as preservatives in a capsule formulation by HPLC using the Purospher® STAR, RP-8 endcapped, 250 x 4.6 mm, 5 μm column.
Section Overview

Figure 1.B vitamins and its preservatives.
Components in capsule sample (indicated by the manufacturer) |
|---|
Experimental
Standard & Sample Preparation
Diluent: Water:glacial acetic acid:acetonitrile (89:1:10 v/v)
Standard Solution for System Suitability
Weigh and transfer 6 mg of thiamine, riboflavin and pyridoxine, 15 mg of nicotinamide, 2 mg of methyl paraben and propyl paraben each into separate 10 mL volumetric flask. Add 5 mL of glacial acetic acid to each flask and sonicate for 10 minutes. Make up to volume with diluent. Further transfer 1 mL of each solution of thiamine, riboflavin and pyridoxine, 2 mL of nicotinamide, 0.2 mL of methyl paraben and 0.1 mL of propyl paraben into a 20 mL volumetric flask. Add ~10 mL of diluent and sonicate for 10 mins. Make up to volume with diluent. Concentrations in final solution are displayed in Table 1.
Stock Solution Preparation for Accuracy Spiking
Weigh and transfer the reference standard (Table 1) to a 10 mL volumetric flask. Add about 5 mL of glacial acetic acid, sonicate for 5 minutes, and make up to volume with diluent.
Test Sample Preparation
Open 10 capsules, pool the contents and mix well. Weigh an equivalent of five capsules into a 200 mL volumetric flask. Add 100 mL of glacial acetic acid and sonicate for about 5 mins. Make up to volume with the diluent and sonicate for another 10 minutes. Filter the solution with 0.45 μm PVDF syringe filter. Dilute 5 mL of this filtered solution to 25 mL with diluent.
Sample Preparation for Accuracy Spiking
Sample Stock (0% Spiking)
Open 10 capsules, pool the contents and mix well. Weigh an equivalent of five capsules into a 250 mL volumetric flask. Add 125 mL of glacial acetic acid and sonicate for about 5 mins. Make up to volume with the diluent and sonicate for another 10 minutes. Filter the solution with 0.45 μm PVDF syringe filter (Sample Stock for Accuracy). Dilute 2 mL of this filtered solution to 25 mL with diluent.
Sample Spiking
- Sample preparation for accuracy 50%: Transfer 2 mL from the sample stock for accuracy to a 25 mL volumetric flask and add about 625 µL of each pyridoxine, thiamine, and riboflavin stock solutions respectively. To it, then add 1875 µL nicotinamide, 250 µL methyl paraben, and 125 µL of propyl paraben and makeup to the volume with diluent and shake thoroughly.
- Sample preparation for accuracy 100%: Transfer 2 mL from the sample stock for accuracy, to 25 mL volumetric flask and add about 1250 µL of each pyridoxine, thiamine, and riboflavin stock solutions respectively. To it, then add 3750 µL nicotinamide, 500 µL methyl paraben, and 250 µL of propyl paraben and makeup to the volume with diluent and shake thoroughly.
- Sample preparation for accuracy 150%: Transfer 2 mL from the sample stock for accuracy to a 25 mL volumetric flask and add about 1875 µL of each pyridoxine, thiamine, and riboflavin stock solutions respectively. To it, then add 5625 µL nicotinamide, 750 µL methyl paraben, and 375 µL of propyl paraben and makeup to the volume with diluent and shake thoroughly.
Analysis by HPLC-UV
Standards and samples were analyzed using conditions described in Table 3.
System Suitability Criteria – Standard Solution
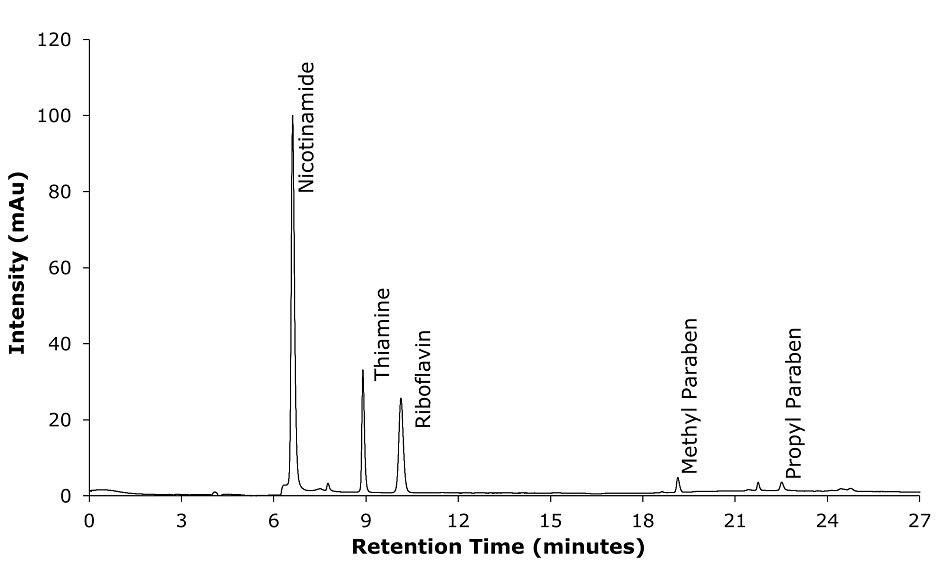
Figure 2.Chromatogram of standards solution for nicotinamide, thiamine, riboflavin, methyl paraben, propyl paraben with detection at 254 nm
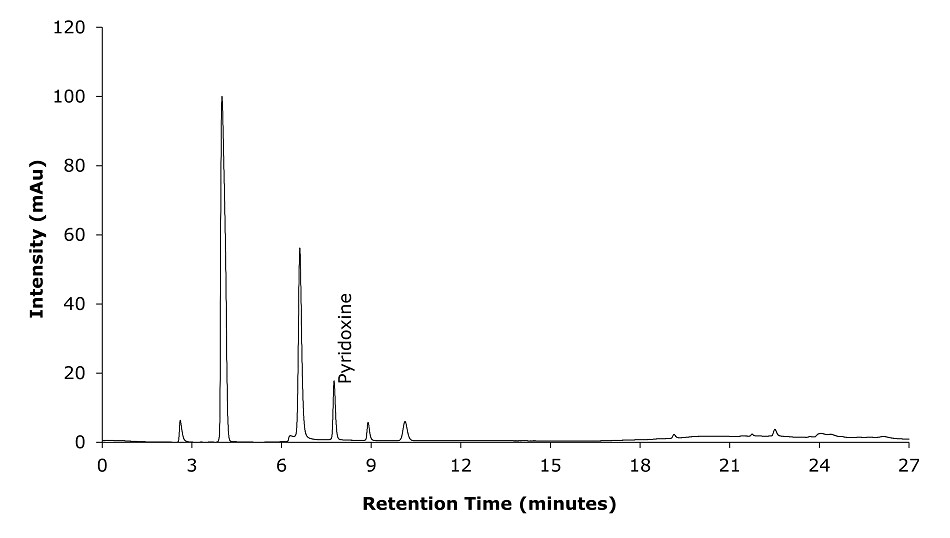
Figure 3.Chromatogram of standards solution with detection at 210 nm for pyridoxine determination.
Sample Suitability Criteria – Test Solution
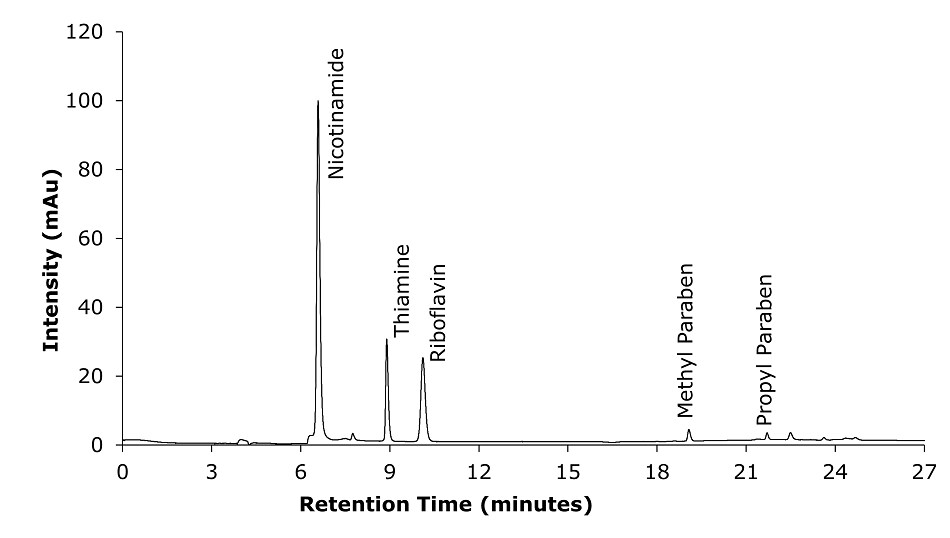
Figure 4.Chromatogram Test Solution (capsules) for 5 of the analytes at 254 nm.
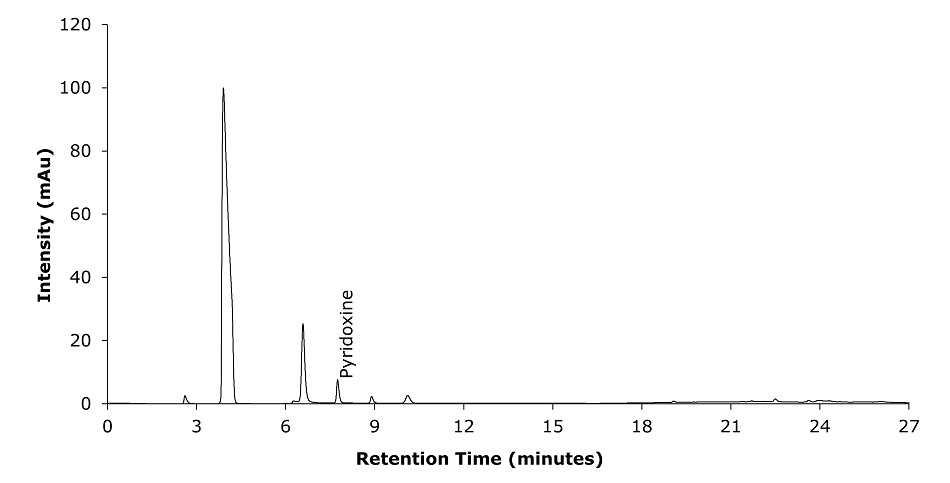
Figure 5.Chromatogram Test Solution (capsules) at 254 nm for pyridoxine of the analytes.
% Recovery of Spiked B Vitamins and Preservatives over Multivitamin Capsule Sample
During accuracy study, spiking of vitamins over the sample solution led to saturation of the detector response, so while performing accuracy spiking study, it became necessary to reduce the sample concentration of the base sample (Sample Stock for Accuracy- disolved into 250 mL instead if 200 mL as for the test sample) and then proceed with and spiking of vitamin standards and preservatives at the Accuracy Levels of 50%, 100% and 150% of the actual working level over the reduced base sample solution concentration.
The results for the recovery determination using the overspiked multivitamin capsule samples are shown in Tables 12 to 17. The recoveries ranged from 96.0 to 110%.
Conclusion
An HPLC method for the quantification of B vitamins and preservatives using the Purospher® STAR RP-8 endcapped (RP-8e) column was developed and evaluated. Using 2 wave lengths in the UV detection with the described conditions, all components in the capsule sample could be separated with a resolution of >2.
The method for the injected solutions was determined to be linear over a concentration range of (LOQ to highest calibrator) 2.89 to 45 µg/mL for thiamine, 3.74 to 45 µg/mL pyridoxine and 6.47 to 45 µg/mL riboflavin and, 21.34 to 225 µg/mL for nicotinamide. The methyl and propyl parabens preservatives have a linear concentration range of 0.74 µg/mL to 3 µg/mL and 0.19 to 1.5 µg/mL respectively. The % RSD for the system precision of each component was <2%.
The limit of detection (LOD) for the B vitamins ranged from 0.95 to 7.04 µg/mL and for methyl and propyl paraben, it was 0.24 and 0.06 µg/mL respectively. The limit of quantification (LOQ) for the B vitamins ranged from 2.89 to 21.34 µg/mL and for methyl and propyl paraben, it was 0.74 and 0.19 µg/mL respectively. The percentage recovery of the B vitamins and parabens ranged from 96% to 110%.
See more applications for Food & Beverage testing.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?