Asymmetric Transfer Hydrogenation
Josephine Nakhla
chemfiles volume 9 article 2
The use of transfer hydrogenation to reduce alkenes, carboxyl groups, ketones, or imines has become very popular. Hashiguchi et al. reported the asymmetric transfer hydrogenation of ketones using a catalyst system comprised of ruthenium complexed with a chiral diamine ligand (RuCl(p-cymene)[(S,S)-Ts-DPEN], Scheme 5).1 Subsequently, this methodology was extended to include transfer hydrogenation of imines with low catalyst loadings, good yields, and excellent enantioselectivities of the desired products observed (Schemes 6 and 7). A variety of imines were subjected to these reaction conditions, with slight variations in the catalyst-ligand composition and/or solvent, leading to excellent yields and enantioselectivities of the functionalized amine heterocycles.2
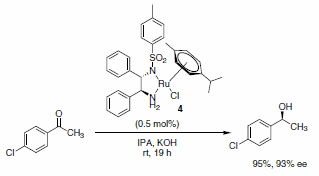
Scheme 5.
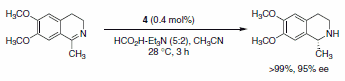
Scheme 6.

Scheme 7.
Matsumura and co-workers also accomplished the asymmetric transfer hydrogenation of α,β-acetylenic ketones using the RuCl(p-cymene) [(R,R)-Ts-DPEN] catalyst system. As shown in Scheme 8, the reduction to the propargylic alcohols occurs selectively without any competitive reaction with the alkynes.3
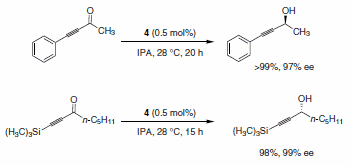
Scheme 8.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?