Performing a Purification of IgG Antibodies with Protein G HP SpinTrap™/Ab SpinTrap™
Protein G HP SpinTrap™ are prepacked, single-use spin columns for protein enrichment of target antigens from antibody-antigen complexes of monoclonal and polyclonal antibodies from unclarified serum and cell culture supernatants (Figure 3.7). In addition, the columns are designed for small-scale purification of multiple samples in parallel and are suitable for use in antibody screening experiments. Protein G HP SpinTrap™ contains Protein G Sepharose® High Performance, which has a high protein binding capacity, and is compatible with all buffers commonly used in antibody purification.
The 16 columns supplied in each package can be used with a standard microcentrifuge and one purification run takes less than 20 min.
Each package of Protein G HP SpinTrap™ contains a protocol for protein enrichment of target antibody-antigen complexes by immunoprecipitation and a protocol for antibody purification.
Ab SpinTrap™ is another pack size of Protein G HP SpinTrap™ columns (Figure 3.8); 50 columns are provided with Ab SpinTrap™.

Figure 3.7.Protein G HP SpinTrap™ columns are used for protein enrichment of target proteins using antibodies bound to the protein G ligand and are also used for small-scale purification of antibodies.
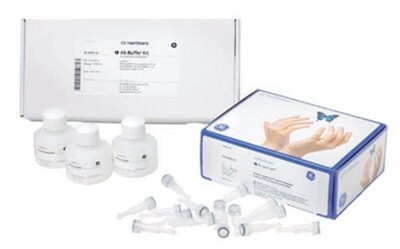
Figure 3.8.Ab SpinTrap™ columns and Ab Buffer Kit combine to allow rapid, small-scale purification of antibodies using a microcentrifuge.
Purification of antibodies from serum without sample clarification, dilution, or filtration is possible with Protein G HP SpinTrap™ column and Ab SpinTrap™ columns. Using these columns, loss of target protein caused by manual operations such as sample centrifugation, transfer of sample to centrifuge tubes, and collecting supernatant is minimized. Figure 3.9 shows the result of purification of anti-HSA (human serum albumin, lane 2 of SDS gel) using Ab SpinTrap™ column from the undiluted serum of an immunized rabbit.

Figure 3.9.SDS-PAGE (reducing conditions; ExcelGel™ SDS Gradient 8–18; Coomassie™ Blue staining) of eluted pool of purified anti-HSA in undiluted serum from an immunized rabbit.
General handling of SpinTrap™ columns
Lids and bottom caps: Lids and bottom caps are used during the incubation and elution but not during equilibration and washing. Before centrifugation, remove the bottom cap and slightly open the screw cap lid (twist the cap lid ~ 90° counterclockwise).
Bottom cap removal: Twist the bottom cap off the SpinTrap™ column before dispensing liquid into the column. Remember to save the bottom cap.
Incubation: Make sure that the medium is fully suspended before incubating with end-over-end mixing. All incubations should normally be performed at room temperature. However, incubations may be performed at lower temperatures when a slower process is preferable.
After centrifugation: Immediately after centrifugation, re-insert the bottom cap into the bottom of the SpinTrap™ column (before the incubation and elution steps).
Liquid collection: After each step, place the SpinTrap™ column in a fresh 2 mL microcentrifuge tube (not included) for liquid collection.
Elution: For the elution steps, mix by manually inverting the SpinTrap™ column.
Sample preparation
Refer to Desalting and buffer exchange for general considerations.
The sample should have a pH around 7 before applying to the column. If required, adjust sample conditions to the pH and ionic strength of the binding buffer by either buffer exchange on a desalting column (see Desalting and buffer exchange) or dilution and pH adjustment.
Buffer preparation
Binding buffer: 0.02 M sodium phosphate, pH 7.0
Elution buffer: 0.1 M glycine-HCl, pH 2.7
Neutralizing buffer: 1 M Tris-HCl, pH 9.0
Water and chemicals used for buffer preparation should be of high purity. Filter buffers through a 0.45 µm filter before use.
Buffers can be prepared from the 10× stock solutions of binding and elution buffers supplied with Ab Buffer Kit (GE28903059).
Purification
- Prepare two collection tubes per sample for eluted fractions, each containing 30 µL neutralizing buffer.
To preserve the activity of acid-labile IgG, we recommend adding 30 µL of 1 M Tris-HCl pH 9.0 to collection tubes, which ensures the final pH of the sample will be approximately neutral.
- Invert and shake the column repeatedly to resuspend the medium. Remove the bottom cap from the column. Save the bottom cap. Centrifuge for 30 s at 70 to 100 × g to remove the storage solution.
- Equilibrate by adding 600 µL binding buffer, centrifuge for 30 s at 70 to 100 × g.
- Bind antibody by adding max. 600 µL of antibody sample. Secure the top cap tightly and incubate for 4 min while gently mixing. Centrifuge for 30 s at 70 to 100 × g.
Several sample applications can be made subsequently as long as the capacity of the column is not exceeded.
- Wash by adding 600 µL binding buffer, centrifuge for 30 s at 70 to 100 × g.
- Add 400 µL of elution buffer and mix by inversion. Place the column in a 2 mL microcentrifuge tube containing 30 µL neutralizing buffer (step 1). Elute by centrifugation for 30 s at 70 × g and collect the eluate.
- Place the column in a new 2 mL microcentrifuge tube containing 30 µL neutralizing buffer (step 1). Centrifuge for 30 s at 70 × g and collect the second eluate. Most of the bound antibody is eluted after two elution steps.
Desalt and/or transfer purified IgG fractions to a suitable buffer using a desalting column (see Desalting and buffer exchange).
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?