USP HPLC Organic Impurity Analysis for Lamotrigine Tablets (SST) on an Ascentis® Express C18
Materiales
Estándar
columna analítica
componente de la fase móvil
prep. de la muestra
CONDITIONS
column
Ascentis® Express C18, 250 x 4.6 mm I.D., 5 µm (50538-U)
mobile phase
Acetonitrile:methanol:buffer (10:30:60) v/v/v; Buffer– 0.8 g/L of ammonium acetate, adjusted with glacial acetic acid to a pH of 4.5
gradient
Isocratic
flow rate
1 mL/min
pressure
216 bar (3132 psi)
column temp.
Ambient
detector
UV, 210 nm
injection
5 µL
sample/matrix
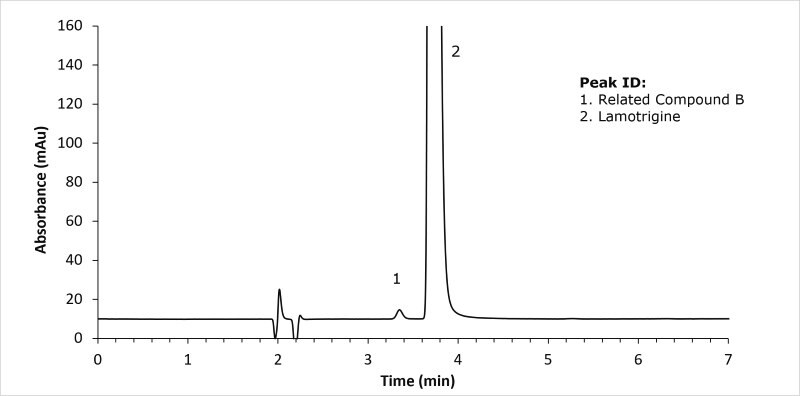
System suitability solution - 1.0 µg/mL of lamotrigine related compound B and 0.4 mg/ml of lamotrigine in methanol and buffer (60:40) v/v
Descripción
Descripción general
Lamotrigine is an anti-convulsant medication used to treat epilepsy and to delay or prevent the recurrence of depressive episodes in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome.
In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression, but for patients with bipolar disorder who are not currently symptomatic, it appears to be effective in reducing the risk of future episodes of depression.
This application illustrated the system suitability for the organic impurity analysis for lamotrigine tablets testing following the currently official United States Pharmacopoeia monograph
Nota de análisis
For the lamotrigine standard solution, the RSD was 0.43 (USP specification NMT 10% RSD) and the tailing factor for lamotrigine was 1.7 (USP specification NMT 2). For the displayed system suitability solution the resolution between lamotrigine and the related compound B was 2.6 (USP specification NLT 2.0).
Otras notas
App_367I
Información legal
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany