M55909
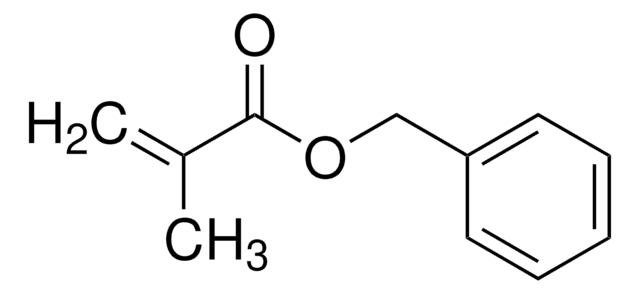
Methyl methacrylate
contains ≤30 ppm MEHQ as inhibitor, 99%
Synonym(s):
Methacrylic acid methyl ester, Methyl 2-methylprop-2-enoate, Methyl 2-methylpropenoate
About This Item
Recommended Products
vapor density
3.5 (vs air)
Quality Level
vapor pressure
29 mmHg ( 20 °C)
Assay
99%
autoignition temp.
815 °F
contains
≤30 ppm MEHQ as inhibitor
expl. lim.
12.5 %
refractive index
n20/D 1.414 (lit.)
bp
100 °C (lit.)
mp
−48 °C (lit.)
density
0.936 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
COC(=O)C(C)=C
InChI
1S/C5H8O2/c1-4(2)5(6)7-3/h1H2,2-3H3
InChI key
VVQNEPGJFQJSBK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Lanthanide-complex grafted poly(methyl methacrylate-co-maleic anhydride) copolymer. These luminescent polymers exhibit high thermal stability and can be used as luminous layers for optoelectronic devices.
- Poly (methyl methacrylate) (PMMA), is a common material used in the production of lenses for concentrating photovoltaic (CPV) modules.
- Polymethyl methacrylate, methyl methacrylate crosspolymer, and methyl methacrylate/glycol dimethacrylate crosspolymers. These polymers are used in cosmetic surgery, dentistry, and joint replacement.
- Poly (methyl methacrylate) (PMMA)-based personalized medical devices.
- Interpenetrating methyl methacrylate-based polymeric networks with enhanced thermal and mechanical properties.
- Poly(methyl methacrylate-co-hydroxyethyl methacrylate) (PMMA-co-PHEMA) copolymers by emulsion copolymerization. These copolymers form thermooxidatively stable and ductile films.
- Poly(methyl methacrylate) nanoparticles through differential microemulsion polymerization.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
50.0 °F - closed cup
Flash Point(C)
10 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Protocols
ARGET ATRP procedure facilitates PMMA polymer brush growth with surface cleaning and initiator monolayer deposition.
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
RAFT polymerization offers precise control, enabling tailored synthesis of complex polymer structures.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service