P25485
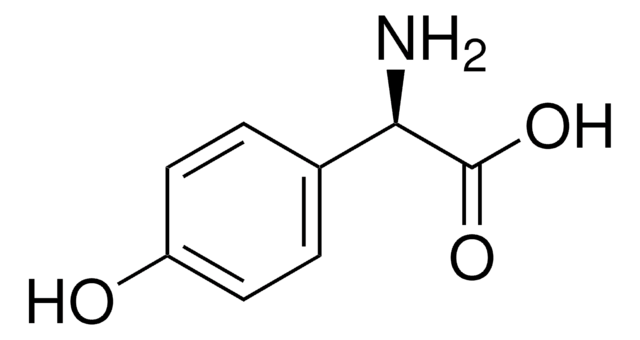
D−(−)-α-Phenylglycine
99%, detection
Synonym(s):
(R)-(−)-2-Phenylglycine, D-2-Phenylglycine, R-(−)-α-Aminophenylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
151.16
Beilstein:
2208676
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
D−(−)-α-Phenylglycine, 99%
Quality Level
Assay
99%
form
powder or crystals
optical activity
[α]20/D −155°, c = 1 in 1 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
302 °C (dec.) (lit.)
application(s)
detection
SMILES string
N[C@@H](C(O)=O)c1ccccc1
InChI
1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m1/s1
InChI key
ZGUNAGUHMKGQNY-SSDOTTSWSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyung Min Kim et al.
The Journal of chemical physics, 128(4), 041104-041104 (2008-02-06)
Photo-oxidation of amino acids is known to generate reactive protein radicals that lead to lethal disorders. We investigated photoionization of hydrated phenylglycine complexes in the gas phase and found that the excess internal energy from photoionization drives decarboxylation in competition
Yvonne J Mast et al.
Journal of biotechnology, 155(1), 63-67 (2010-12-15)
Pristinamycin I (PI), a streptogramin type B antibiotic produced by Streptomyces pristinaespiralis, contains the aproteinogenic amino acid L-phenylglycine. Recent sequence analysis led to the identification of a set of putative phenylglycine biosynthetic genes. Successive inactivation of the individual genes resulted
R S Kulkarni et al.
Hindustan antibiotics bulletin, 47-48(1-4), 41-44 (2008-08-14)
The parameters for complete hydrolysis of L-phenyl acetyl phenylglycine (L-PAPG) using immobilized penicillin G acylase (IMEPGA) were investigated. IMEPGA exhibited maximum activity at pH 8.5 and 50 degrees C. The apparent Km value observed was 10 mM. Quantitative hydrolysis (>97%)
Antonella Caputo et al.
The Journal of pharmacology and experimental therapeutics, 330(3), 783-791 (2009-06-06)
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channel. The mutations G551D and G1349D, which affect the nucleotide-binding domains (NBDs) of CFTR protein, reduce channel activity. This defect can be corrected pharmacologically
Aaron D Mills et al.
Bioorganic & medicinal chemistry letters, 20(1), 87-91 (2009-12-04)
A developing therapy of cystic fibrosis caused by the DeltaF508 mutation in CFTR employs correction of defective CFTR chloride channel gating by a 'potentiator' and of defective CFTR protein folding by a 'corrector'. Based on SAR data for phenylglycine-type potentiators
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service