361763
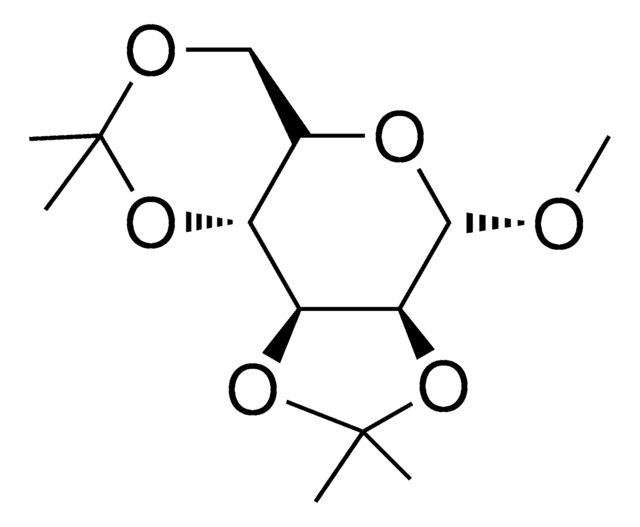
2,3:5,6-Di-O-isopropylidene-α-D-mannofuranose
97%
Synonym(s):
D-Mannose diacetonide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H20O6
CAS Number:
Molecular Weight:
260.28
Beilstein:
84382
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
optical activity
[α]20/D +23°, c = 1 in acetone
mp
125-126 °C (dec.) (lit.)
SMILES string
CC1(C)OC[C@@H](O1)[C@H]2OC(O)[C@@H]3OC(C)(C)O[C@@H]23
InChI
1S/C12H20O6/c1-11(2)14-5-6(16-11)7-8-9(10(13)15-7)18-12(3,4)17-8/h6-10,13H,5H2,1-4H3/t6?,7-,8+,9?,10?/m1/s1
InChI key
JWWCLCNPTZHVLF-RGJLLFKYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Protected mannose intermediate used in the syntheses of ovalicin and of the sugar core of hikizimycin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shunya Takahashi et al.
The Journal of organic chemistry, 70(24), 10162-10165 (2005-11-19)
[reaction: see text] A new synthesis of epoxyketone 22 is described that is a key intermediate in Barton's synthesis of ovalicin (2), a powerful anti-angiogenetic inhibitor. The key process for the construction of 22 was ring-closing metathesis of olefins 11
Furstner, A. et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 12, 76-76 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(3AR,5S,6S,6AR)-5-((R)-2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)-2,2-DIMETHYLTETRAHYDROFURO[3,2-D][1,3]DIOXOL-6-OL AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/241/825/1c695a85-5c36-42d3-806a-30876a4dabac/640/1c695a85-5c36-42d3-806a-30876a4dabac.png)