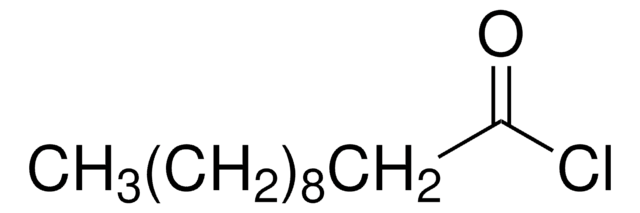468479
4-Pentenoyl chloride
98%
Synonym(s):
4-Pentenoic acid chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2C=CHCH2CH2COCl
CAS Number:
Molecular Weight:
118.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
125 °C (lit.)
density
1.074 g/mL at 25 °C (lit.)
SMILES string
ClC(=O)CCC=C
InChI
1S/C5H7ClO/c1-2-3-4-5(6)7/h2H,1,3-4H2
InChI key
JDKQTIKEGOOXTJ-UHFFFAOYSA-N
General description
4-Pentenoyl chloride has been identified as a key impurity in 5-chlorovaleroyl chloride (5-CVC). 4-Pentenoyl chloride can be synthesized by reacting thionyl chloride and 4-pentenoic acid.
Application
4-Pentenoyl chloride may be used in the preparation of:
- D-pro-L-derived cyclic peptides
- 4-pentenoylcobalt tricarbonyl
- N-4-pentenoyl-L-cysteine methyl ester
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
80.6 °F - closed cup
Flash Point(C)
27 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Nageshwara Rao et al.
The Journal of organic chemistry, 69(6), 2181-2184 (2004-04-03)
An acyclic tripeptide based on a heterochiral D-pro-L-pro template shows a propensity to exist as a 3(10) helical conformation and can be cyclized, via ring-closing metathesis, to the corresponding cyclic tetrapeptides without disrupting the helical conformations in CDCl(3) as well
Alkyl-and Acyl-cobalt Carbonyls Containing Olefinic Unsaturation. Allylcobalt Tricarbonyl and Related Compounds1.
Heck RF and Breslow DS.
Journal of the American Chemical Society, 83(5), 1097-1102 (1961)
Synthesis and radical polyaddition of optically active monomers derived from cysteine.
Kudo H, et al.
Macromolecules, 32(25), 8370-8375 (1999)
Liya Tang et al.
Journal of pharmaceutical and biomedical analysis, 53(3), 309-314 (2010-05-05)
5-Chlorovaleroyl chloride (5-CVC) is commonly used as an alkylating agent in the synthesis of pharmaceutical intermediates, active ingredients, as well as other specialty chemicals. It is critical to monitor the impurities present in 5-CVC as they may have a direct
Norlaily Ahmad et al.
Biomacromolecules, 20(7), 2506-2514 (2019-06-28)
Inflammatory conditions are frequently accompanied by increased levels of active proteases, and there is rising interest in methods for their detection to monitor inflammation in a point of care setting. In this work, new sensor materials for disposable single-step protease
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service