1463600
USP
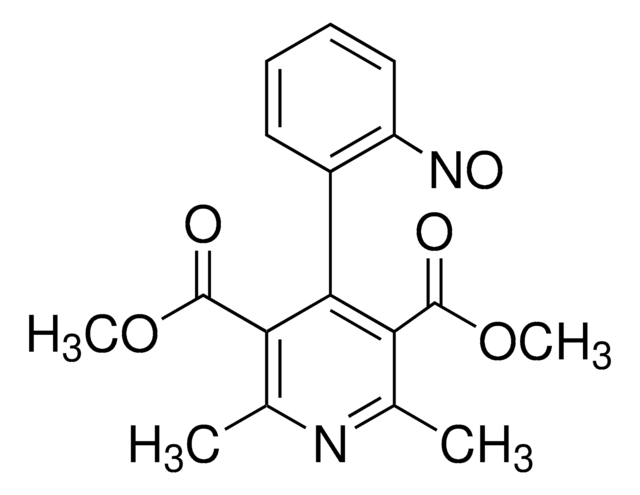
Nifedipine Nitrophenylpyridine Analog
United States Pharmacopeia (USP) Reference Standard
别名:
Oxidized Nifedipine, 2,6-Dimethyl-4-(2´-nitrophenyl)-3,5-pyridinecarboxylic acid dimethyl ester
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C17H16N2O6
CAS号:
分子量:
344.32
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
nifedipine
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
COC(=O)c1c(C)nc(C)c(C(=O)OC)c1-c2ccccc2[N+]([O-])=O
InChI
1S/C17H16N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8H,1-4H3
InChI 密鑰
UMQHJQGNGLQJPF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Nifedipine Nitrophenylpyridine Analog USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Nifedipine Capsules
- Nifedipine
- Nifedipine Extended-Release Tablets
生化/生理作用
CYP3A4 nifedipine metabolite. Nifedipine (parent compound) is an antianginal and antihypertensive agent.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Wenzhan Yang et al.
Current drug discovery technologies, 5(2), 129-139 (2008-08-05)
This study reports the use of para-sulphonato calix[8]arene to produce stable complexes with improved bioavailability for nifedipine, a calcium-channel blocker that is practically insoluble in water. Thermal analysis and electrospray ionisation mass spectroscopy confirmed that nifedipine formed complexes with the
Tsai-Shin Chiang et al.
PloS one, 9(4), e94885-e94885 (2014-04-16)
Human hepatoma cell lines are commonly used as alternatives to primary hepatocytes for the study of drug metabolism in vitro. However, the phase I cytochrome P450 (CYP) enzyme activities in these cell lines occur at a much lower level than
Camille C Savary et al.
Drug metabolism and disposition: the biological fate of chemicals, 42(8), 1235-1240 (2014-05-17)
Humans are usually exposed to several pesticides simultaneously; consequently, combined actions between pesticides themselves or between pesticides and other chemicals need to be addressed in the risk assessment. Many pesticides are efficient activators of pregnane X receptor (PXR) and/or constitutive
Yohei Kosugi et al.
Xenobiotica; the fate of foreign compounds in biological systems, 45(4), 345-352 (2014-11-12)
1. The purpose of this study was to clarify species differences in the heteroactivation of CYP3A substrates by efavirenz, which is known from clinical studies to activate midazolam 1'-hydroxylation, and to assess the feasibility of an animal model. 2. In monkey and
Yoshihiko Shimokawa et al.
Biological & pharmaceutical bulletin, 37(11), 1727-1735 (2014-11-05)
Delamanid is a new drug for the treatment of multidrug-resistant tuberculosis. Individuals who are co-infected with human immunodeficiency virus and Mycobacterium tuberculosis may require treatment with a number of medications that might interact significantly with the CYP enzyme system as
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门