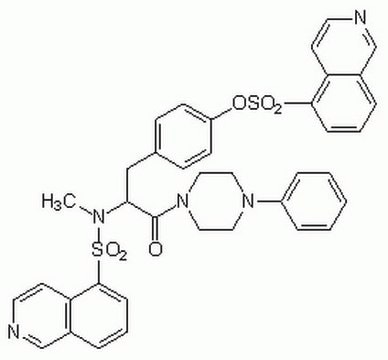
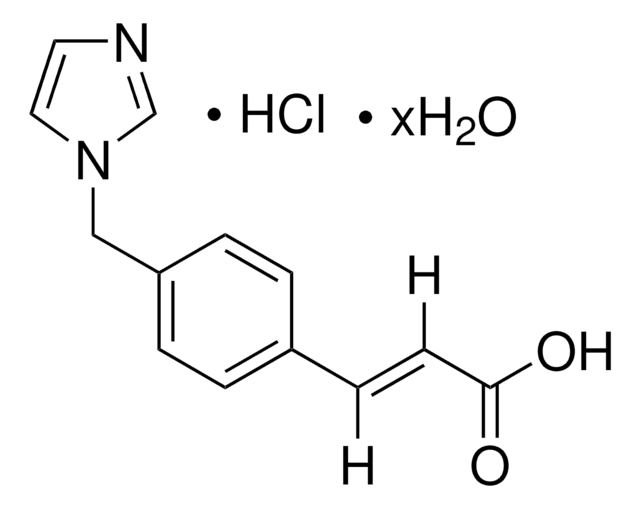
推荐产品
化驗
≥95% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to beige
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
−20°C
SMILES 字串
CCCCC1=CC=C(C(C)=C1)/N=C/NO
InChI
1S/C12H18N2O/c1-3-4-5-11-6-7-12(10(2)8-11)13-9-14-15/h6-9,15H,3-5H2,1-2H3,(H,13,14)
InChI 密鑰
LYNOGBKNFIHKLE-UHFFFAOYSA-N
生化/生理作用
HET0016 is a potent and selective inhibitor against cytochrome P450 (CYP450) ω-hydroxylases (human CYP4A11/4A22/4F2/4F3, rat CYP4A1/4A2/4A3/4A8)-mediated 20-hydroxyeicosatetraenoic acid (20-HETE) biosynthesis (IC50 = 8.9/35 nM using human/rat renal microsomes), exhibiting much reduced potency against epoxyeicosatrienoic acids (EETs) biosynthesis (IC50 = 2.8 μM/rat microsome) or the enzyme activities of CYP2C9/2D6/3A4 and COX (IC50 = 3.3/83.9/71 μM and 2.3 μM). HET0016 is widely employed both in cultures (1-10 μM) and in aminals in vivo (1-10 mg/kg via i.v., i.m., i.p. or s.c.) for probing 20-HETE-dependent physiological and pathological processes.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Manoocher Soleimani et al.
PloS one, 11(7), e0159804-e0159804 (2016-07-22)
Contribution of salt wasting and volume depletion to the pathogenesis of hypercalciuria and hyperphosphaturia is poorly understood. Pendrin/NCC double KO (pendrin/NCC-dKO) mice display severe salt wasting under basal conditions and develop profound volume depletion, prerenal renal failure, and metabolic alkalosis
N Miyata et al.
British journal of pharmacology, 133(3), 325-329 (2001-05-26)
The present study examined the inhibitory effects of N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016) on the renal metabolism of arachidonic acid by cytochrome P450 (CYP) enzymes. HET0016 exhibited a high degree of selectivity in inhibiting the formation of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) in rat renal
Yeon Jung Choi et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 112, 205-215 (2018-01-07)
This study was designed to characterize lauric acid metabolism to facilitate the establishment of cytochrome P450 4A11 (CYP4A11) inhibition assay. Three metabolites (2-, 11-, and 12-hydroxylauric acids) were identified in pooled human liver microsomes based on comparisons with authentic standards.
M Sato et al.
Bioorganic & medicinal chemistry letters, 11(23), 2993-2995 (2001-11-21)
N-(4-Butyl-2-methylphenyl)-N'-hydroxyformamidine (HET0016) was evaluated as the first potent and selective inhibitor of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) synthase. The IC(50) value of HET0016 for the production of 20-HETE from arachidonic acid (AA) by human renal microsomes was 8.9+/-2.7 nM, with over 200
Guangrui Lai et al.
Prostaglandins & other lipid mediators, 134, 123-130 (2017-08-16)
We previously found that 20-hydroxyeicosatetraeonic acid (20-HETE) showed an effect on proteasome activity in cytochrome P450 F2 (CYP4F2) transgenic mice. Proteasome subunit β5 (PSMB5) is a primary subunit of the proteasome. In the current study, we examine whether 20-HETE has
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门