所有图片(1)
About This Item
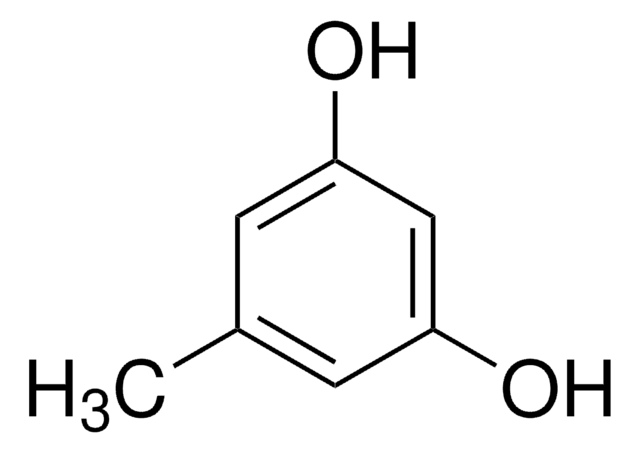
经验公式(希尔记法):
C10H14O2
CAS号:
分子量:
166.22
Beilstein:
1942645
MDL號碼:
分類程式碼代碼:
85151701
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
analytical standard
品質等級
化驗
≥97.0% (GC)
儲存期限
limited shelf life, expiry date on the label
應用
cleaning products
cosmetics
food and beverages
personal care
格式
neat
SMILES 字串
OC1=C(CCCC)C=CC(O)=C1
InChI
1S/C10H14O2/c1-2-3-4-8-5-6-9(11)7-10(8)12/h5-7,11-12H,2-4H2,1H3
InChI 密鑰
CSHZYWUPJWVTMQ-UHFFFAOYSA-N
一般說明
4-n-butylresorcinol is a derivative of resorcinol and a potent human tyrosinase inhibitor. It may be used to decrease skin irritation and is also known to inhibit melanin production.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
293.0 °F
閃點(°C)
145 °C
William Wargniez et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 1060, 416-423 (2017-07-05)
In the present study, three laboratories independently compared percutaneous absorption and distribution of 4-n-butylresorcinol, using human skin from five donors. Each laboratory used the same protocol for percutaneous absorption studies but different LC-MS/MS analytical methods to quantify the test compound.
The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: a randomized controlled split-face trial.
Huh SY, et al.
Annals of Dermatology, 22(1), 21-25 (2010)
S-J Lee et al.
International journal of cosmetic science, 39(3), 248-255 (2016-10-27)
4-n-butylresorcinol is a competitive inhibitor of tyrosinase and has been used as an antimelanogenic agent. However, its inhibition mechanism in intact cells is not fully understood. To elucidate the cellular mechanism, we compared in vitro and in vivo inhibitory effects
Justyna Odrobińska et al.
Polymers, 12(2) (2020-02-09)
A novel initiator, bromoester modified 4-n-butylresorcinol (4nBREBr2), was prepared and utilized in controlled atom transfer radical polymerization (ATRP) to obtain three series of amphiphilic copolymers. The V-shaped copolymers of methyl methacrylate (MMA), 2-hydroxyethyl methacrylate (HEMA), and poly(ethylene glycol) methyl ether
Justyna Odrobińska et al.
Materials (Basel, Switzerland), 13(16) (2020-08-09)
The amphiphilic copolymers of poly(ethylene glycol) methyl ether methacrylate (MPEGMA) and alkyne functionalized 2-hydroxyethyl methacrylate (AlHEMA) were synthesized by controlled atom transfer radical polymerization (ATRP). The reactions were carried out using the standard ATRP initiator ethyl α-bromoisobutyrate, (EiBBr) and the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门