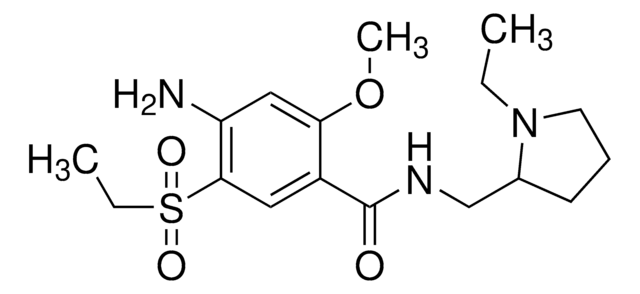
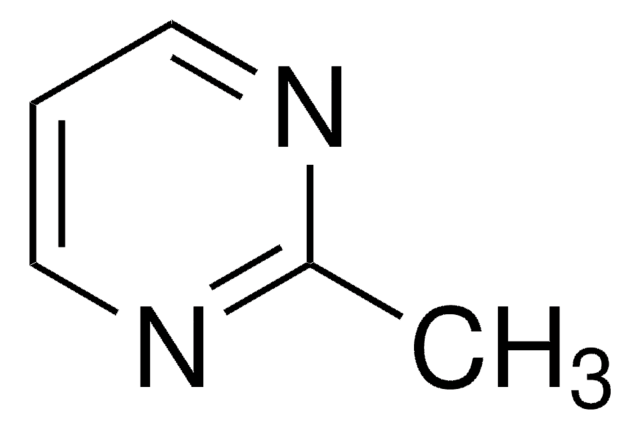
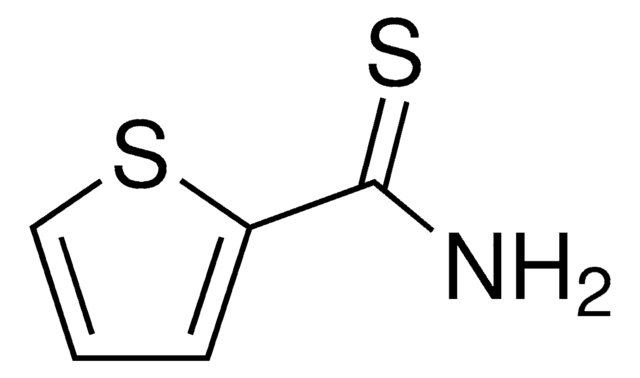
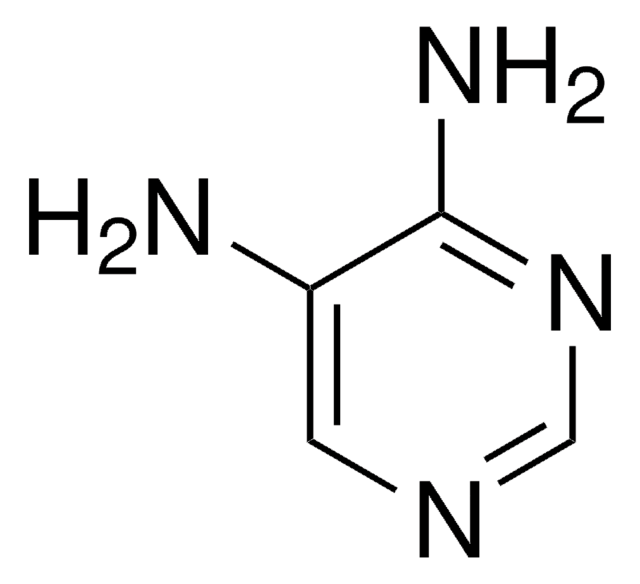
About This Item
推荐产品
品質等級
化驗
95%
形狀
liquid
反應適用性
reaction type: click chemistry
折射率
n/D 1.407
密度
1.310 g/L at 25 °C
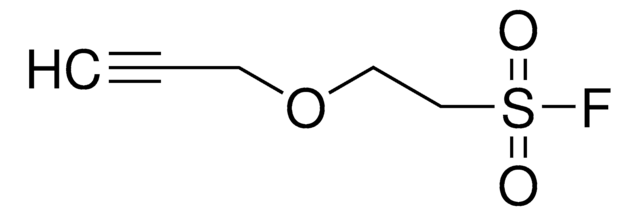
SMILES 字串
FS(C1CC1)(=O)=O
InChI
1S/C3H5FO2S/c4-7(5,6)3-1-2-3/h3H,1-2H2
InChI 密鑰
XPOPREDCDIARJH-UHFFFAOYSA-N
應用
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
148.0 °F
閃點(°C)
64.44 °C
商品
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
相关内容
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门