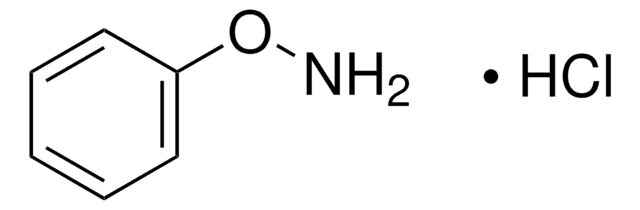
推荐产品
化驗
≥95.0%
形狀
solid
mp
80-84 °C
儲存溫度
−20°C
SMILES 字串
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
InChI 密鑰
CKRZKMFTZCFYGB-UHFFFAOYSA-N
應用
N-苯基羟胺作为起始材料,可用于下列合成反应:
- 通过连续3,3-重排和环脱水反应,用金催化剂与脂肪族末端炔烃进行处理,合成2-烷基吲哚。
- 通过三组分一锅催化法,与醛和 α, β不饱和醛合成异恶唑烷。
- 通过homo3+2偶极环加成反应,处理醛和环丙烷,合成四氢1,2-恶嗪。
訊號詞
Danger
危險聲明
防範說明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
Wang Y, et al.
Chemical Communications (Cambridge, England), 47(27), 7815-7817 (2011)
Copper-catalyzed amination of alkenes and ketones by phenylhydroxylamine.
Ho C-M and Lau T-C
New. J. Chem., 24(11), 859-863 (2000)
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
Lloyd J Nadeau et al.
Applied and environmental microbiology, 69(5), 2786-2793 (2003-05-07)
Hydroxylamino aromatic compounds are converted to either the corresponding aminophenols or protocatechuate during the bacterial degradation of nitroaromatic compounds. The origin of the hydroxyl group of the products could be the substrate itself (intramolecular transfer mechanism) or the solvent water
C C Somerville et al.
Journal of bacteriology, 177(13), 3837-3842 (1995-07-01)
Pseudomonas pseudoalcaligenes JS45 grows on nitrobenzene as a sole source of carbon, nitrogen, and energy. The catabolic pathway involves reduction to hydroxylaminobenzene followed by rearrangement to o-amino-phenol and ring fission (S. F. Nishino and J. C. Spain, Appl. Environ. Microbiol.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门