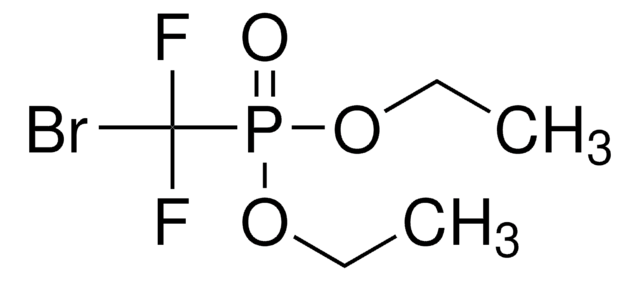
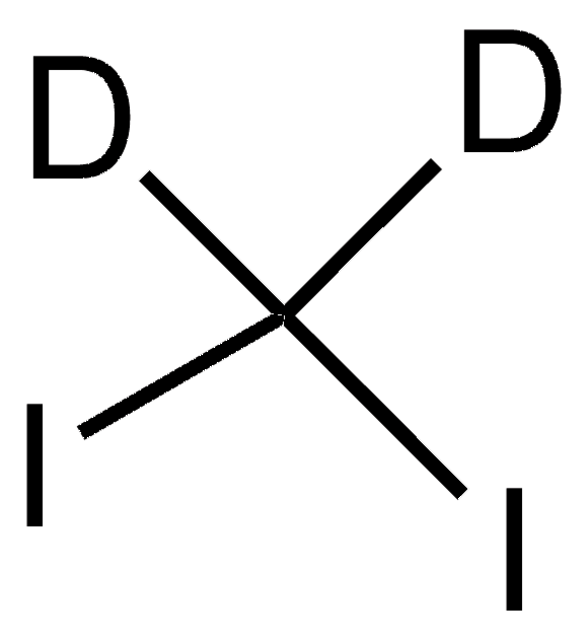
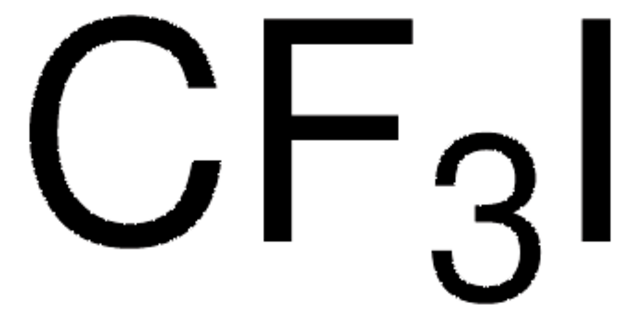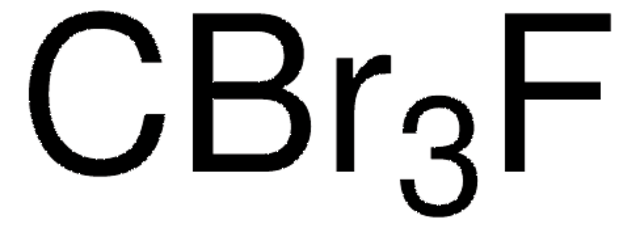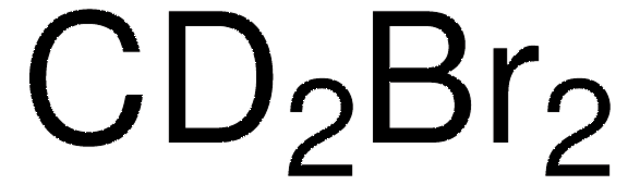推荐产品
蒸汽密度
7.24 (vs air)
蒸汽壓力
12.79 psi ( 20 °C)
化驗
≥95.0% (GC)
形狀
liquid
折射率
n20/D 1.398 (lit.)
n20/D 1.398
bp
22-23 °C (lit.)
mp
−142-−141 °C (lit.)
密度
2.297 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
FC(F)(Br)Br
InChI
1S/CBr2F2/c2-1(3,4)5
InChI 密鑰
AZSZCFSOHXEJQE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
The photolysis products of dibromodifluoromethane were characterized by matrix isolation infrared and UV/Visible spectroscopy. Free radical addition of dibromodifluoromethane with fluoroolefins has been reported. Dibromodifluoromethane reacts with hydrocarbon olefins to yield 1,3-dibromo-1,1-difluoroalkene. Sulfinatodehalogenation reagent promoted addition reaction of difluorodibromomethane with alkenes, alkynes and cyclic enol ethers has been described.
應用
- Synthesis of 3‑Fluoropyrazoles via Radical-Polar Crossover Photoinduced Cyclization: This study highlights the use of dibromodifluoromethane (CF2Br2) for the synthesis of valuable difluorinated and monofluorinated compounds through a novel photoinduced cyclization process (MA Reed, ND Patil, Synfacts, 2023).
其他說明
作为反应物与羰基化合物通过 Wittig 烯化反应转化成二氟亚甲基或氟亚甲基衍生物;二氟卡宾的前体
訊號詞
Warning
危險分類
Eye Irrit. 2 - Ozone 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Gloves
其他客户在看
Free Radical Additions Involving Fluorine Compounds. I. The Addition of Dibromodifluoromethane to Hydrocarbon Olefins.
Tarrant P and Lovelace AM.
Journal of the American Chemical Society, 76(13), 3466-3468 (1954)
H.P. Fritz et al.
Zeitschrift fur Naturforschung, B: Chemical Sciences, 86, 1375-1375 (1981)
Free Radical Additions Involving Fluorine Compounds. IV. The Addition of Dibromodifluoromethane to Some Fluoroolefins1.
Tarrant P, et al.
Journal of the American Chemical Society, 77(10), 2783-2787 (1955)
J.S. Houlten et al.
Tetrahedron, 49, 8087-8087 (1993)
Lisa George et al.
The Journal of chemical physics, 132(8), 084503-084503 (2010-03-03)
The photolysis products of dibromodifluoromethane (CF(2)Br(2)) were characterized by matrix isolation infrared and UV/Visible spectroscopy, supported by ab initio calculations. Photolysis at wavelengths of 240 and 266 nm of CF(2)Br(2):Ar samples (approximately 1:5000) held at approximately 5 K yielded iso-CF(2)Br(2)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门