所有图片(1)
About This Item
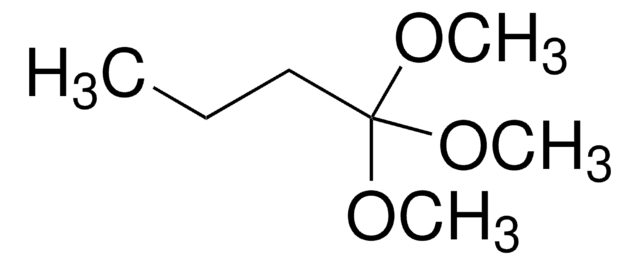
线性分子式:
CH3CH2CH2CH2C(OCH3)3
CAS号:
分子量:
162.23
Beilstein:
1739200
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
折射率
n20/D 1.410 (lit.)
bp
164-166 °C (lit.)
密度
0.941 g/mL at 25 °C (lit.)
SMILES 字串
CCCCC(OC)(OC)OC
InChI
1S/C8H18O3/c1-5-6-7-8(9-2,10-3)11-4/h5-7H2,1-4H3
InChI 密鑰
XUXVVQKJULMMKX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Kinetics of unimolecular gas-phase elimination of trimethyl orthovalerate has been investigated.
應用
Trimethyl orthovalerate has been used in the preparation of:
- various 9-O-acyl derivatives of N-acetyl- and N-glycoloyl-neuraminic acid
- 2-methyl, 2-ethyl, 2-propyl and 2-butyl-3-benzimidazolyl-4(3H)-quinazolinones
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
107.6 °F - closed cup
閃點(°C)
42 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Edgar Marquez et al.
The journal of physical chemistry. A, 114(12), 4203-4209 (2010-03-10)
The rates of gas-phase elimination of trimethyl orthovalerate and trimethyl orthochloroacetate have been determined in a static system, and the reaction Pyrex vessels have been deactivated with the product of decomposition of allyl bromide. The reactions are unimolecular and follow
A facile synthesis of new 3-(2-benzimidazolyl)-2-alkyl-4-(3H)-quinazolinones under microwave irradiation.
Hazarkhani H and Karimi B.
Tetrahedron, 59(26), 4757-4760 (2003)
H Ogura et al.
Carbohydrate research, 167, 77-86 (1987-09-15)
Various 9-O-acyl derivatives of N-acetyl- and N-glycoloyl-neuraminic acid, and O-(5-acetamido-3,5-dideoxy-D-glycero-alpha- and beta-D-galacto-2-nonulopyranosylonic acid)-(2----6)-O-beta-D-galactopyranosyl-(1----4)-D-glucopyranose were regioselectively synthesized by use of ortho esters. In addition, 5-acetamido-4-O-acetyl-D-glycero-D-galacto-2-nonulopyranosonic acid was prepared starting from the benzyl and methyl esters of N-acetylneuraminic acid.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门