推荐产品
化驗
97%
mp
102-106 °C (lit.)
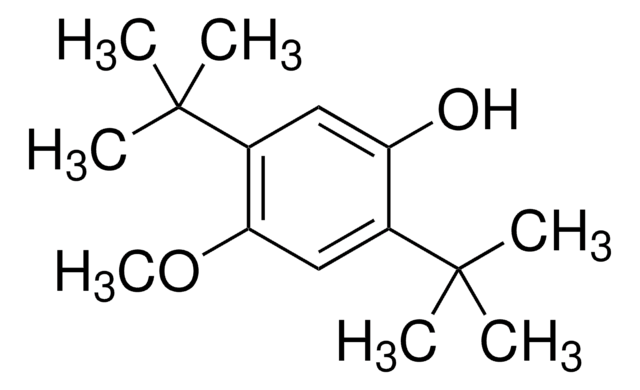
SMILES 字串
COc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-8-10(17-7)9-12(13(11)16)15(4,5)6/h8-9,16H,1-7H3
InChI 密鑰
SLUKQUGVTITNSY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,6-Di-tert-butyl-4-methoxyphenol is a phenolic antioxidant. It participates in In(trifluoromethanesulfonate)3-catalyzed tandem reaction of ortho-alkynylarylimine with various nucleophiles.
應用
2,6-Di-tert-butyl-4-methoxyphenol has been used to protect cosmetics, drugs and foods from oxidative degradation.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
In(OTf)3-catalyzed tandem nucleophilic addition and cyclization of ortho-alkynylarylaldimines to 1,2-dihydroisoquinolines.
Reiko Yanada et al.
Angewandte Chemie (International ed. in English), 45(23), 3822-3825 (2006-05-04)
Leonid L Chepelev et al.
The Journal of organic chemistry, 71(1), 22-30 (2006-01-04)
[reaction: see text] Rate constants for hydrogen-atom transfer (HAT) from bilirubin dimethyl ester (BRDE) and biliverdin dimethyl ester (BVDE) to peroxyl radicals during inhibited autoxidation of styrene initiated by azo-bisisobutyronitrile (AIBN) were k(inh)(BRDE) = 22.5 x 10(4) and k(inh)(BVDE) =
M E Hidalgo et al.
Phytochemistry, 37(6), 1585-1587 (1994-12-01)
The antioxidant activity of lichenic metabolites, depsides and depsidones, was assessed by their effects as inhibitors of rat brain homogenate auto-oxidation and beta-carotene oxidation. The results obtained in both systems indicate that lichenic metabolites afford a moderate protection in the
Murat Sentürk et al.
Bioorganic & medicinal chemistry, 17(8), 3207-3211 (2009-02-24)
The inhibition of two human cytosolic carbonic anhydrase (hCA, EC 4.2.1.1) isozymes I and II, with a series of phenol derivatives was investigated by using the esterase assay, with 4-nitrophenyl acetate as substrate. 2,6-Dimethylphenol, 2,6-diisopropylphenol (propofol), 2,6-di-t-butylphenol, butylated hydroxytoluene, butylated
Christopher Elam et al.
European journal of medicinal chemistry, 46(5), 1512-1523 (2011-03-01)
Two screening protocols based on recursive partitioning and computational ligand docking methodologies, respectively, were employed for virtual screens of a compound library with 345,000 entries for novel inhibitors of the enzyme sarco/endoplasmic reticulum calcium ATPase (SERCA), a potential target for
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门