推荐产品
化驗
98%
形狀
solid
折射率
n20/D 1.4763 (lit.)
bp
233 °C (lit.)
mp
33-36 °C (lit.)
溶解度
ethanol: soluble 5%, clear to slightly hazy, colorless to dark yellow
儲存溫度
2-8°C
SMILES 字串
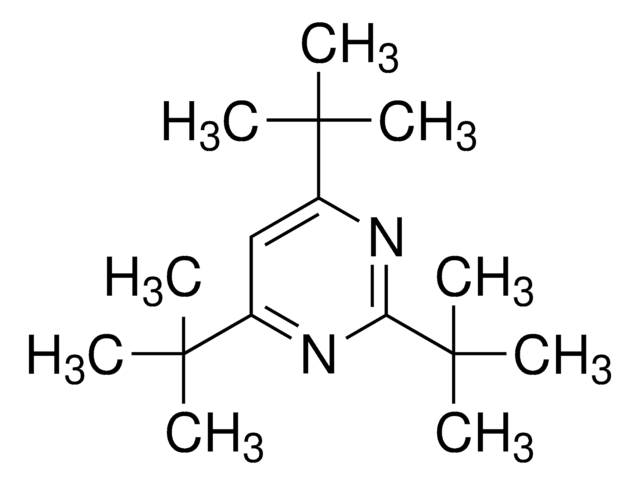
Cc1cc(nc(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C14H23N/c1-10-8-11(13(2,3)4)15-12(9-10)14(5,6)7/h8-9H,1-7H3
InChI 密鑰
HVHZEKKZMFRULH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,6-二-叔t-丁基-4-甲基吡啶是一种位阻型非亲和碱,可区分布仑斯惕(质子)酸与路易斯酸。它也可直接将乙醛和酮高产率转化成乙烯基三氟甲磺酸酯。它可抑制GaCl3-催化苯酚邻乙基化反应中产物的脱硅和水合作用。
應用
2,6-二- 叔 -丁基-4-甲基吡啶用于:
- 1,2-二氢-2-硅杂萘衍生物的合成
- 高炔丙基叠氮衍生物在 PtCl 4 催化下的环化反应
- β-硫代吡喃核苷的非对映选择性合成
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
183.2 °F - closed cup
閃點(°C)
84 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Hidekazu Arii et al.
Chemical communications (Cambridge, England), 50(50), 6649-6652 (2014-05-16)
Treatment of dialkylbenzylsilane (1) with trityl tetrakis(pentafluorophenyl)borate (TPFPB) in the presence of terminal or internal alkynes (3) and 2,6-di-tert-butyl-4-methylpyridine gave the corresponding 1,2-dihydro-2-silanaphthalene derivatives (4) in 34-82% yields.
Organic Syntheses, 68, 138-138 (1990)
Katsumi Kobayashi et al.
Journal of the American Chemical Society, 124(29), 8528-8529 (2002-07-18)
Phenols are ethynylated at the ortho position with silylated chloroethyne in the presence of a catalytic amount of GaCl3 and lithium phenoxide. The lithium salt is essential for the catalysis, and addition of 2,6-di(tert-butyl)-4-methylpyridine inhibits desilylation and hydration of the
Synthesis, 283-283 (1980)
Daichi Nakamura et al.
Organic & biomolecular chemistry, 17(14), 3581-3589 (2019-03-23)
Regioselectivity of Ln(OTf)3-catalysed alcoholysis of 2,3- and 3,4-epoxy alcohols was closely investigated to expand the scope of the transformations. The synthetic use was demonstrated by application to the construction of 4-propoxy-5-hydroxy-2,3-pentanedione (C4-propoxy-HPD), which is a potent synthetic mediator in AI-2
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门