所有图片(1)
About This Item
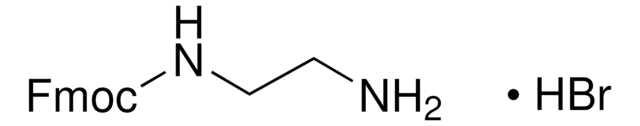
线性分子式:
(CH)3COCONH(CH2)5NH2
CAS号:
分子量:
202.29
Beilstein:
3603658
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥97.0% (NT)
反應適用性
reagent type: cross-linking reagent
折射率
n20/D 1.460
密度
0.972 g/mL at 20 °C (lit.)
官能基
Boc
amine
SMILES 字串
NCCCCCNC(OC(C)(C)C)=O
InChI
1S/C10H22N2O2/c1-10(2,3)14-9(13)12-8-6-4-5-7-11/h4-8,11H2,1-3H3,(H,12,13)
InChI 密鑰
DPLOGSUBQDREOU-UHFFFAOYSA-N
應用
Some of the reported applications of N-Boc-cadaverine include:
- Synthesis of of a supermacrocycle that self-assemble to form organic nanotubes.
- Preparation of water-soluble unsymmetrical sulforhodamine fluorophores from monobrominated sulfoxanthene dye.
- Synthesis of functionalized porphyrins as biocompatible carrier system for photodynamic therapy (PDT).
其他說明
制备聚胺和聚酰胺的结构单元
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
228.2 °F - closed cup
閃點(°C)
109.0 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
C Melchiorre et al.
Journal of medicinal chemistry, 32(1), 79-84 (1989-01-01)
Several polymethylene tetraamines related to methoctramine (1) were prepared and evaluated for their blocking activity on M-2 muscarinic receptors in guinea pig atria and ileum. It turned out that antimuscarinic potency depends on the following parameters: (a) nature of the
T. Teshima et al.
Tetrahedron, 47, 3305-3305 (1991)
Helical rosette nanotubes: design, self-assembly, and characterization.
Fenniri H, et al.
Journal of the American Chemical Society, 123(16), 3854-3855 (2001)
A toolset of functionalized porphyrins with different linker strategies for application in bioconjugation.
Staegemann M H, et al.
Organic & Biomolecular Chemistry, 14(38), 9114-9132 (2016)
Rapid Synthesis of Unsymmetrical Sulforhodamines Through Nucleophilic Amination of a Monobrominated Sulfoxanthene Dye.
Chevalier A, et al.
European Journal of Organic Chemistry, 2015(1), 152-165 (2015)
商品
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门











![2-[2-[(叔丁氧羰基)氨基]乙氧基]乙醇 97%](/deepweb/assets/sigmaaldrich/product/structures/413/416/884359e5-1cb4-4071-bb4f-28d9844db662/640/884359e5-1cb4-4071-bb4f-28d9844db662.png)
