推荐产品
化驗
97%
形狀
solid
bp
284 °C (lit.)
mp
56-58 °C (lit.)
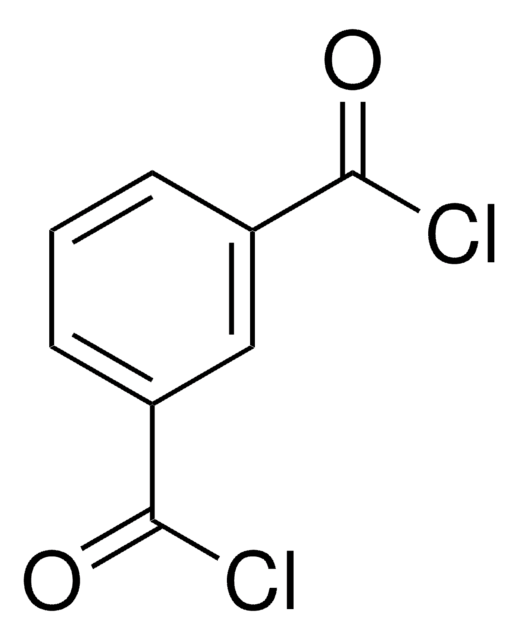
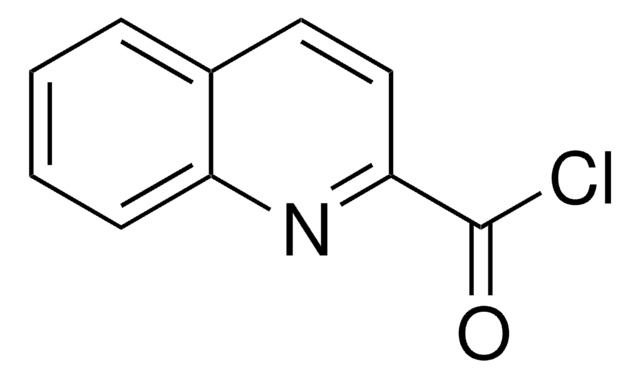
SMILES 字串
ClC(=O)c1cccc(n1)C(Cl)=O
InChI
1S/C7H3Cl2NO2/c8-6(11)4-2-1-3-5(10-4)7(9)12/h1-3H
InChI 密鑰
GWHOGODUVLQCEB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2.6-吡啶二羰基二氯化物可用作合成以下物质的起始材料:
- 吡啶基聚酰胺聚酯,与手性二氢溴化二胺反应形成光学活性大环化合物。
- 吡啶桥联 2,6-双-甲酰胺席夫′碱,采用L-丙氨酸或2- 甲基-丙氨酸甲酯进行处理。
- N,N′-双(1-芘甲基)吡啶-2,6-二羧酰胺,在存在碱的条件下与1-芘甲基胺盐酸盐反应。
訊號詞
Warning
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Synthesis of some new pyridine-2, 6-carboxamide-derived Schiff bases as potential antimicrobial agents
Al-Omar MA and Amr Abd El-Galil E
Molecules (Basel), 15(7), 4711-4721 (2010)
Mohamed A Al-Omar et al.
Molecules (Basel, Switzerland), 15(7), 4711-4721 (2010-07-27)
A series of pyridine-bridged 2,6-bis-carboxamide Schiff's bases has been prepared starting from 2,6-pyridinedicarbonyl dichloride (1) and L-alanine or 2-methyl-alanine methyl ester.The coupling of acid chloride 1 with L-alanine methyl ester hydrochloride -or 2-methylalanine methyl ester hydrochloride gave the corresponding 2,6-bis-carboxamide
H Zhao et al.
The Journal of organic chemistry, 65(10), 2933-2938 (2000-05-18)
Five new chiral macrocycles, 3a-e, have been prepared by the acylation cyclization of chiral diamine dihydrobromide intermediates 2a-c with 2,6-pyridinedicarbonyl dichloride in highly diluted solution at room temperature. The chiral diesters 1a-c needed for the preparation of the macrocycles were
Recognition of sequence-information in synthetic copolymer chains by a conformationally-constrained tweezer molecule
Colquhoun HM, et al.
Faraday Discussions, 143(7), 205-220 (2009)
Synthesis and characterization of pyridine-based polyamido-polyester optically active macrocycles and enantiomeric recognition for D-and L-amino acid methyl ester hydrochloride
Zhao H and Hua W
The Journal of Organic Chemistry, 65(10), 2933-2938 (2000)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门