推荐产品
蒸汽壓力
4.67 psi ( 20 °C)
品質等級
化驗
98%
形狀
liquid
自燃溫度
527 °F
折射率
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
密度
0.824 g/mL at 25 °C (lit.)
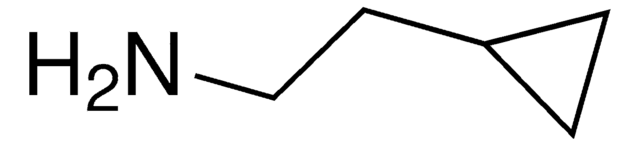
SMILES 字串
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
InChI 密鑰
HTJDQJBWANPRPF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
环丙胺 (CPA) 用于 N -\ [4-(4-氟)苯基-2-氨基噻唑-5-基] 嘧啶-2-基-烷基胺衍生物的合成 。已用于合成 Pt (CPA) 2 ( 双甲硫亚甲基丙二酸酯)和 Pt (CPA) 2 (双 乙基硫亚甲基丙二酸酯)配合物 。
生化/生理作用
环丙胺通过一种机制使细胞色素 P450 酶失活,该机制包括在氮气中先发生单电子氧化,然后环丙烷环断裂,导致酶的共价修饰 。它是 脱氮副球菌 中一种基于机制的喹啉甲胺脱氢酶抑制剂。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
33.8 °F - closed cup
閃點(°C)
1 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles
其他客户在看
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Coordination Mode vs. Anticancer Activity of the Platinum (II) Complexes Involving Sulfur-Containing Ylidenemalonate Ligands.
Sakai N, et al.
Bull. Korean Chem. Soc., 19(12), 1377-1379 (1998)
Catherine A Faler et al.
Organic letters, 9(10), 1987-1990 (2007-04-24)
An intermolecular Ti(IV)-mediated cyclopropanation reaction has been used to synthesize substituted 2-phenylcyclopropylamines and constrained analogues of the neurotransmitters histamine and tryptamine. Many hydroxy- and methoxy-substituted phenylcyclopropylamines are known to inhibit monoamine oxidase and have been shown to mimic hallucinogens. These
Jo-Yanne Le Berre et al.
PloS one, 12(12), e0190341-e0190341 (2017-12-28)
Little is known about the responses of plant roots to filamentous pathogens, particularly to oomycetes. To assess the molecular dialog established between the host and the pathogen during early stages of infection, we investigated the overall changes in gene expression
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门