8.09622
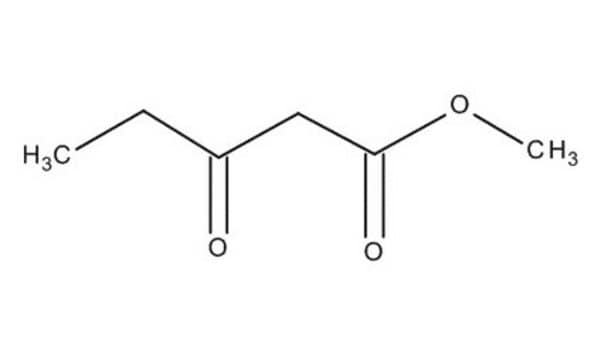
Ethyl acetoacetate
for synthesis
Synonym(s):
Ethyl acetoacetate, EAA, Acetoacetic acid ethyl ester
About This Item
Recommended Products
vapor pressure
0.26 hPa ( 20 °C)
Quality Level
Assay
≥98.0% (GC)
form
liquid
autoignition temp.
350 °C
potency
3980 mg/kg LD50, oral (Rat)
>5000 mg/kg LD50, skin (Rabbit)
expl. lim.
1.0-54 % (v/v)
pH
4.0 (20 °C, 110 g/L in H2O)
mp
-53.3 °C
transition temp
flash point 73.5 °C
solubility
130.3 g/L
density
1.03 g/cm3 at 20 °C
storage temp.
2-30°C
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Application
- 7-hydroxycoumarin derivatives via Pechmann condensation reaction with 1,3-dihydroxybenzene in the presence of acid catalysts.
- 2,6-disubstituted piperidine alkaloid, (−)-pinidinone via stereoselective α-aminoallylation followed by Grubbs′ olefin cross-metathesis reaction.
- Michael addition products via Michael addition reaction with chalcones and azachalcones in the presence of a base catalyst.
- α, β-unsaturated carbonyl compounds via Knoevenagel condensation reaction with glyceraldehyde acetonide in the presence of a base catalyst.
It can be also utilized as a reactant in the transesterification and asymmetric hydrogenation reactions to produce valuable products.
Analysis Note
Density (d 20 °C/ 4 °C): 1.028 - 1.030
Identity (IR): passes test
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
164.3 °F - closed cup
Flash Point(C)
73.5 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service