684163
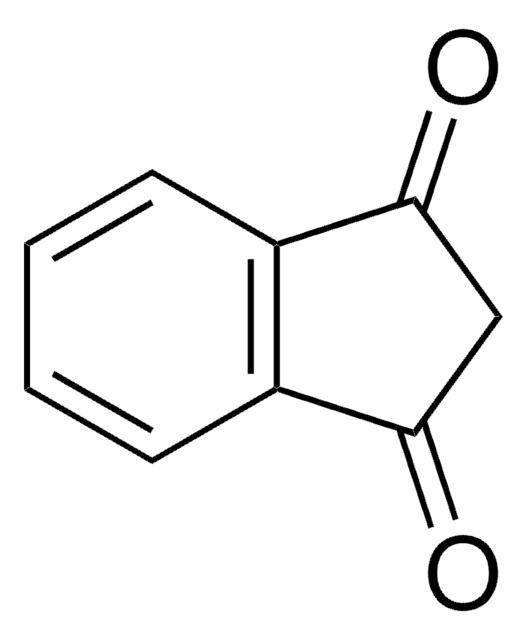
Indan-1,2-dione
97%
Synonym(s):
1H-Indene-1,2(3H)-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H6O2
CAS Number:
Molecular Weight:
146.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
117-124 °C
SMILES string
O=C1Cc2ccccc2C1=O
InChI
1S/C9H6O2/c10-8-5-6-3-1-2-4-7(6)9(8)11/h1-4H,5H2
InChI key
WFFZGYRTVIPBFN-UHFFFAOYSA-N
Application
Indan-1,2-dione is a latent fingerprint reagent widely used in forensic chemistry. It can also be used to synthesize polycyclic arene and heteroarene systems containing five membered rings.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Optimisation and evaluation of 1,2-indanedione for use as a fingermark reagent and its application to real samples.
Wallace-Kunkel C, et al.
Forensic Science International, 168(1), 14-26 (2007)
Patrick Fritz et al.
Forensic science international, 257, 20-28 (2015-08-19)
Dimethylaminocinnamaldehyde was re-evaluated as a wet contact reagent for the treatment of latent fingermarks on porous substrates. A new formulation (consisting of 0.028 g p-dimethylaminocinnamaldehyde, 0.84 mL glacial acetic acid, 6.2 mL ethyl acetate and 0.993 L 40-60 °C petroleum
Chemical development of latent fingerprints: 1, 2-indanedione has come of age.
Wiesner S, et al.
Journal of Forensic Sciences, 46(5), 1082-1084 (2001)
A Combined Experimental and Theoretical Study of the Ammonium Bifluoride Catalyzed Regioselective Synthesis of Quinoxalines and Pyrido[2,3-b]pyrazines.
Lassagne F, et al.
Synthesis, 47, 2680-2689 (2015)
Niko Nicolasora et al.
Forensic science international, 288, 266-277 (2018-05-25)
This paper contains details of work carried out to identify the most effective processing conditions for the optimized 1,2-indandione/zn formulation developed for use under UK conditions. Using direct measurements of fluorescence taken from test spots of amino acids and eccrine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service