B22984
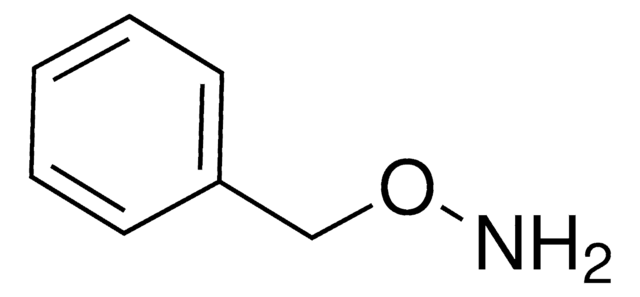
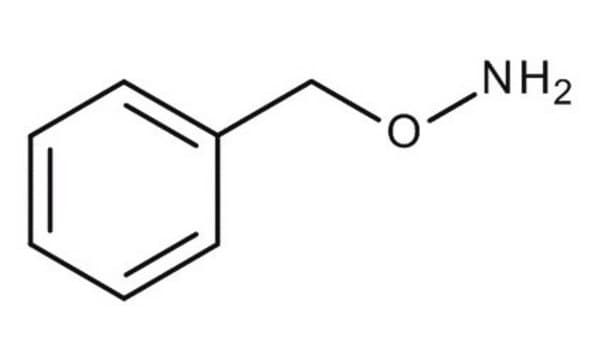
O-Benzylhydroxylamine hydrochloride
99%
Synonym(s):
Benzyloxyamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2ONH2 · HCl
CAS Number:
Molecular Weight:
159.61
Beilstein:
3687991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
238 °C (subl.) (lit.)
SMILES string
Cl.NOCc1ccccc1
InChI
1S/C7H9NO.ClH/c8-9-6-7-4-2-1-3-5-7;/h1-5H,6,8H2;1H
InChI key
HYDZPXNVHXJHBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Effective reagent used to prepare α-hydroxybenzylamines from α-hydroxyketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Stampf et al.
Die Pharmazie, 35(1), 43-44 (1980-01-01)
The study of the blood levels and tissue concentrations in mice to which 14C-benzyloxyamine hydrochloride was applied in the form of a spray and of a suspensoid aerosol evidenced the good abosrption of this pharmacon. Maximum blood levels were observed
S M Breckenridge et al.
Journal of chromatography. B, Biomedical sciences and applications, 694(2), 289-296 (1997-07-04)
Extraction and derivatization of carbonyls to benzyloximes, pentafluorobenzyloximes or 2,4-dinitrophenylhydrazones is simplified and reaction times are substantially reduced by simultaneous sorption and derivatization from aqueous solution onto a solid phase. In this reaction a macroreticular polystyrene-divinylbenzene resin acts as a
A A Purmal et al.
Mutation research, 364(3), 193-207 (1996-12-02)
Duplex oligonucleotides containing the base lesion analogs, O-methylhydroxylamine- and O-benzylhydroxylamine-modified abasic (AP) sites, were substrates for the DNA N-glycosylases endonuclease III, formamidopyrimidine DNA N-glycosylase and T4 endonuclease V. These N-glycosylases are known to have associated AP lyase activities. In contrast
Tetrahedron Letters, 32, 711-711 (1991)
D F Magin
Journal of chromatography, 202(2), 255-261 (1980-12-19)
A qualitative and semi-quantitative method was established for the investigation of low-molecular-weight volatile carbonyl compounds in cigarette whole smoke. The carbonyls were trapped on a silica gel "column" and eluted with water. The aqueous solution was then treated with benzyloxyamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service