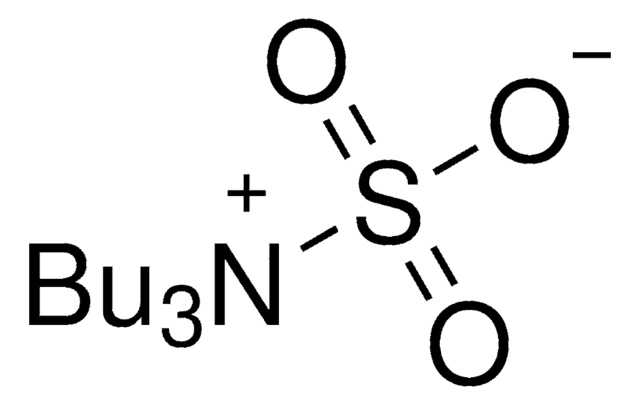135879
Sulfur trioxide trimethylamine complex
Synonym(s):
NSC 9838, Trimethylamine sulfur trioxide complex
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
(CH3)3N · SO3
CAS Number:
Molecular Weight:
139.17
Beilstein:
3681759
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
reaction suitability
reagent type: oxidant
mp
232 °C (dec.) (lit.)
SMILES string
CN(C)C.O=S(=O)=O
InChI
1S/C3H9N.O3S/c2*1-4(2)3/h1-3H3;
InChI key
DXASQZJWWGZNSF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for sulfation reactions involving:
Nucleophile for the synthesis of α-tosyloxy ketones
- Synthesis of sulfate-conjugated resveratrol metabolites
- Chitooligosaccharides used for anti-HIV-1 activity
- Dodecyl thioglycopyranoside used as a surfactant for enantiomeric separation
- Glycosaminoglycans which facilitate activation of signaling pathways dependent on sulfation pattern
- Polysaccharides as heparan sulfate mimetics
Nucleophile for the synthesis of α-tosyloxy ketones
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jure Acimovic et al.
PloS one, 8(7), e68031-e68031 (2013-07-12)
24S- and 27-hydroxycholesterol (24OHC and 27OHC) are potent regulators of different biochemical systems in vitro and are the major circulating oxysterols. A small fraction of these oxysterols has been reported to be sulphated but there are no detailed studies. We
Juliane Strätz et al.
Macromolecular bioscience, 20(2), e1900403-e1900403 (2019-12-31)
Sulfated cellulose (CS) represents an interesting biopolymer due to bioactivity comparable to heparin. However, use of CS for making surface coatings or hydrogels requires the presence of reactive groups for covalent reactions. Here, an approach is presented to oxidize cellulose
Mikael Pedersen et al.
Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment, 34(4), 482-488 (2017-01-21)
After administration of steroids to animals the steroids are partially metabolised in the liver and kidney to phase 2 metabolites, i.e., glucuronic acid or sulphate conjugates. During analysis these conjugated metabolites are normally deconjugated enzymatically with aryl sulphatase and glucuronidase
Shuqian Hu et al.
Carbohydrate polymers, 236, 116052-116052 (2020-03-17)
In this study, a series water soluble sulfate polysaccharides (SPS) with different degrees of substitutions (DS = 0.02∼0.28) were prepared using a linear water-insoluble β-d-(1→3)-glucan. SPS-1, SPS-3 and SPS-7 with substitution degrees of 0.02, 0.06 and 0.25 were used as templates to
Jianhong Yang et al.
Biopolymers, 103(10), 539-549 (2015-04-11)
The 6-amino-6-deoxychitosan (NC) and their 2, 6-di-N-sulfonated derivatives were prepared via N-phthaloylation, tosylation, azidation, hydrazinolysis, reduction of azide groups and N-sulfonation, and their structures were systematically characterized by FT-IR, 2D HSQC NMR, XRD, gel permeation chromatography (GPC), and elemental analysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service