130443
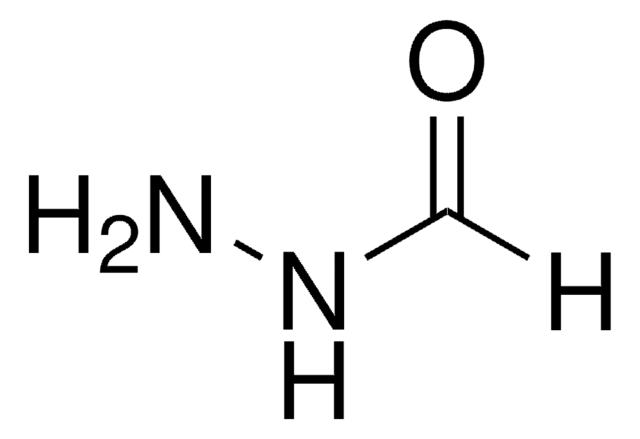
2-Furoic hydrazide
98%
Synonym(s):
2-Furoic acid hydrazide, 2-Furoylhydrazine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O2
CAS Number:
Molecular Weight:
126.11
Beilstein:
114435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
77-79 °C (lit.)
SMILES string
NNC(=O)c1ccco1
InChI
1S/C5H6N2O2/c6-7-5(8)4-2-1-3-9-4/h1-3H,6H2,(H,7,8)
InChI key
SKTSVWWOAIAIKI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Furoic hydrazide (2-Furoylhydrazine) was used to study the Fourier transform infrared spectra, 1H NMR and 13C NMR spectra. It was also used as a reagent for determination of carbohydrates.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Vimalraj et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 78(2), 670-675 (2010-12-31)
The Fourier transform infrared spectra, 1H NMR and 13C NMR spectra of 2-furoic hydrazide have been recorded. Optimized geometry, frequency and intensity of the vibrational bands of 2-furoic hydrazide were obtained by the density functional theory (DFT) and ab initio
M Lever et al.
Analytical biochemistry, 139(1), 205-211 (1984-05-15)
4- Hydroxybenzoylhydrazine ( PAHBAH ) reacts with glucose in hot aqueous solution when alkali exceeds aroylhydrazine concentration. The related 2- furoylhydrazine ( FAH ) reacts at lower alkali concentrations, making this an attractive alternative carbohydrate reagent since it is (unlike
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service