G9793
N-Glycolylneuraminic acid
≥95% (HPLC), semisynthetic
Synonym(s):
Neu5Glc, NeuNGl
About This Item
Recommended Products
biological source
semisynthetic
Assay
≥95% (HPLC)
form
powder
technique(s)
LC/MS: suitable
impurities
water (Karl Fischer)
color
white
solubility
water: soluble 20 mg/mL
suitability
suitable for LC-MS
application(s)
metabolomics
storage temp.
−20°C
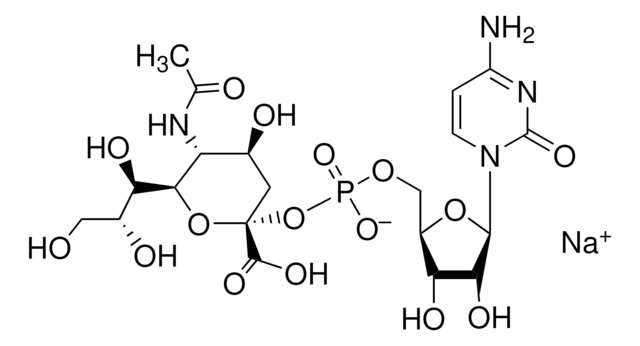
SMILES string
[H][C@]1(O[C@@](O)(C[C@H](O)[C@H]1NC(=O)CO)C(O)=O)[C@H](O)[C@H](O)CO
InChI
1S/C11H19NO10/c13-2-5(16)8(18)9-7(12-6(17)3-14)4(15)1-11(21,22-9)10(19)20/h4-5,7-9,13-16,18,21H,1-3H2,(H,12,17)(H,19,20)/t4-,5+,7+,8+,9+,11-/m0/s1
InChI key
FDJKUWYYUZCUJX-AJKRCSPLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
In humans, the absence of endogenous production results from a gene mutation affecting CMP-Neu5Ac hydroxylase, the enzyme responsible for converting N-acetylneuraminic acid into Neu5Gc. However, Neu5Gc can accumulate in human cells through external ingestion from dietary sources like red meat and dairy products. N-Glycolylneuraminic acid is a versatile compound that finds application in cell biology, metabolomics and biochemical research
Application
- as a sugar in microtiter biofilm methodologic approach for the enhancement of biofilm formation
- as a standard for the determination of sialic acids in the nervous system of silkworm and to find the variations of sialic acids among different developmental stages.
- as a standard in the high-performance liquid chromatography (HPLC) analyses to detect the molecular species of sialic acid (Sia) species using 1,2-diamino-4,5-methylenedioxy-benzene (DMB) as a fluorogenic compound
Biochem/physiol Actions
Features and Benefits
- Ideal for Metabolomics, Biochemical and Cell Biology research
- Versatile and adaptable for wide variety of laboratory and research applications
Other Notes
comparable product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service