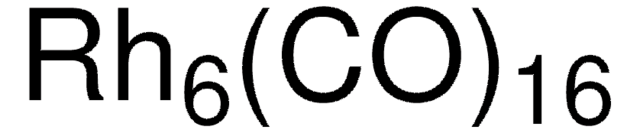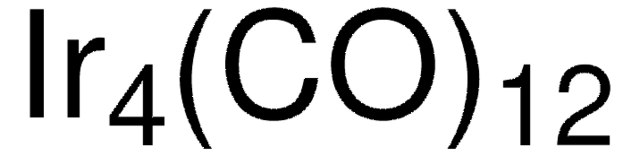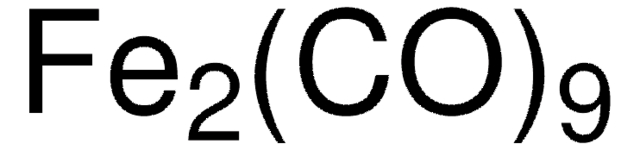245011
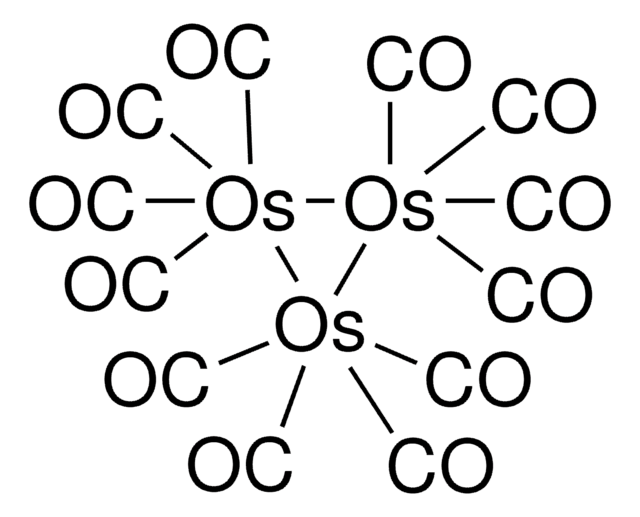
Triruthenium dodecacarbonyl
99%
Synonym(s):
Ruthenium carbonyl, tri-Ruthenium dodecacarbonyl
About This Item
Recommended Products
Quality Level
Assay
99%
form
crystals
reaction suitability
core: ruthenium
reaction type: C-H Activation
reagent type: catalyst
SMILES string
[Ru].[Ru].[Ru].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+]
InChI
1S/12CO.3Ru/c12*1-2;;;
InChI key
NQZFAUXPNWSLBI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Thin film photovoltaic devices have become increasingly important in efficiently harnessing solar energy to meet consumer demand.
Related Content
The Krische group has developed a broad class of “C-C bond forming transfer hydrogenations” wherein the exchange of hydrogen between alcohols and unsaturated reactants serves to generate aldehyde-organometal pairs that combine to give products of carbonyl addition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service