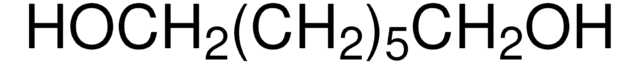H11904
2,5-Hexanediol
99% (mixture of isomers)
Synonym(s):
2,5-Hexylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH2CH2CH(OH)CH3
CAS Number:
Molecular Weight:
118.17
Beilstein:
1719248
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99% (mixture of isomers)
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
216-218 °C (lit.)
density
0.961 g/mL at 25 °C (lit.)
SMILES string
CC(O)CCC(C)O
InChI
1S/C6H14O2/c1-5(7)3-4-6(2)8/h5-8H,3-4H2,1-2H3
InChI key
OHMBHFSEKCCCBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,5-Hexanediol can be used as:
- A reactant to synthesize N-substituted pyrroles by oxidative coupling with primary amines.
- An alkylating agent to synthesize substituted carbazoles via Friedel-Crafts alkylation with indoles.
- A monomer in the preparation of polyesters by reacting with diacid chlorides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Corey Frazer et al.
Nature microbiology, 5(11), 1374-1389 (2020-07-29)
Cell identity in eukaryotes is controlled by transcriptional regulatory networks that define cell-type-specific gene expression. In the opportunistic fungal pathogen Candida albicans, transcriptional regulatory networks regulate epigenetic switching between two alternative cell states, 'white' and 'opaque', that exhibit distinct host
A K Agrawal et al.
Neurotoxicology, 6(3), 53-59 (1985-01-01)
Single exposure of 150, 300 and 600 mg/kg 2,5-hexanediol, (2,5 HD) a metabolite of n-hexane showed no significant effect on 3H-spiroperidol binding to corpus striatal membranes when compared with control animals, where as repeated exposure of 2,5-HD, 300 and 600
On the pattern of changes in the rat nervous system produced by 2,5 hexanediol. A topographical study by light microscopy.
J B Cavanagh et al.
Brain : a journal of neurology, 104(2), 297-318 (1981-06-01)
2,5-Hexane diol induced thymic atrophy and lymphocytotoxicity in rats.
K P Singh et al.
Industrial health, 21(4), 235-242 (1983-01-01)
K Kannan et al.
Environmental research, 36(1), 14-25 (1985-02-01)
A preliminary study on immunotoxicologic evaluation of 2,5-hexanediol (one of the principal metabolites of n-hexane), involving multiple immunological parameters, was carried out in mice. Mice were exposed to 2,5-hexanediol at a 1/5 LD50 dose level for 7 days. Pathotoxicological changes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service