All Photos(1)
About This Item
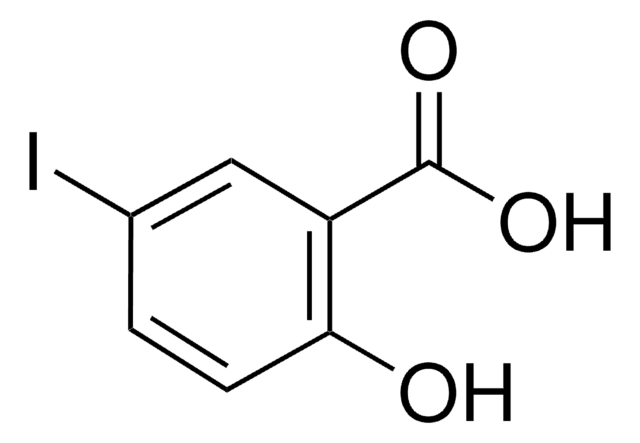
Linear Formula:
2-(HO)C6H4CONH2
CAS Number:
Molecular Weight:
137.14
Beilstein:
742439
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
140-144 °C (lit.)
SMILES string
NC(=O)c1ccccc1O
InChI
1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10)
InChI key
SKZKKFZAGNVIMN-UHFFFAOYSA-N
Gene Information
human ... AR(367)
Looking for similar products? Visit Product Comparison Guide
General description
Salicylamide is used as a starting material for synthesizing substituted salicylamides through acylation reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Patricia D Sauzem et al.
European journal of medicinal chemistry, 43(6), 1237-1247 (2007-09-25)
In this work, we reported the synthesis and evaluation of the analgesic and anti-inflammatory properties of novel 3- or 4-substituted 5-trifluoromethyl-5-hydroxy-4,5-dihydro-1H-1-carboxyamidepyrazoles (where 3-/4-substituent=H/H, Me/H, Et/H, Pr/H, i-Pr/H, Bu/H, t-Bu/H, Ph/H, 4-Br-Ph/H and H/Me) designed in the exploration of the bioisosteric
Jessica Nilsson et al.
Journal of inorganic biochemistry, 105(12), 1795-1800 (2011-11-08)
Reaction of N-(2-hydroxybenzyl)-N-(2-picolyl) glycine (H(2)papy) with VOSO(4) in water gives the oxidovanadium(V) oxido-bridged dimer [{(papy)(VO)}(2) μ-O)] (1). Similarly, reaction of N-(2-hydroxybenzyl) glycine (H(2)glysal) with VOSO(4) gives [(glysal)VO(H(2)O)] (2) and reaction of salicylamide (Hsalam) with VOSO(4) in methanol gives [(salam)(2)VO] (3).
Peter L D Wildfong et al.
Journal of pharmaceutical sciences, 95(12), 2645-2656 (2006-08-23)
The potential for various small molecule organic crystals to undergo complete mechanically induced disordering is investigated. A model is proposed, which considers changes in free energy required for lattice incorporation of a critical dislocation density. Application requires knowledge of a
Yong-Jiang Zhao et al.
International journal of pharmaceutics, 379(1), 90-99 (2009-06-23)
Poly (ethylene glycol)s (PEGs) are potential drug carriers for improving the therapeutic index of anticancer agents. In this work, a novel methodology for constructing PEG prodrug of anthracycline anticancer drugs was developed based on N-Mannich base of salicylamide and its
Zenei Taira et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 42(5), 803-807 (2004-03-30)
To elucidate the effects of Sho-saiko-to extract and its components, baicalin, baicalein, glycyrrhizin and glycyrrhetic acid, against the effects of longer periods of acute hepatic injury induced by CCl4, we measured serum ALT activity in male Wistar rats for five
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service