All Photos(1)
About This Item
Empirical Formula (Hill Notation):
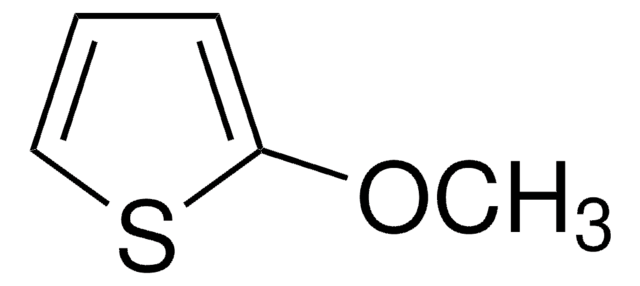
C5H6OS
CAS Number:
Molecular Weight:
114.17
Beilstein:
106404
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.532 (lit.)
bp
80-82 °C/65 mmHg (lit.)
density
1.143 g/mL at 25 °C (lit.)
SMILES string
COc1ccsc1
InChI
1S/C5H6OS/c1-6-5-2-3-7-4-5/h2-4H,1H3
InChI key
RFSKGCVUDQRZSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Methoxythiophene is a thiophene. The intramolecular and intermolecular geometries of crystals of 3-methoxythiophene were studied. Spectroscopic studies of bipolarons derived from oligomerized 3-methoxythiophene in solution has been reported. The electropolymerization of 3-methoxythiophene on Pt and Fe electrodes in an aqueous micellar medium containing sodium dodecyl sulfate and 10-3M bithiophene has been reported. Thin polymer films of 3-methoxythiophene at the cathode in a direct current discharge have been prepared.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
120.2 °F - closed cup
Flash Point(C)
49 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrosynthesis and characterization of poly (3-methoxythiophene)-polybithiophene composite films prepared in micellar media on Pt and Fe substrates.
Dieng MaM, et al.
Physical Chemistry Chemical Physics, 1(8), 1731-1734 (1999)
Martina Marinelli et al.
Chirality, 32(12), 1361-1376 (2020-11-17)
Novel optically active oligothiophenes bearing electron-donating chiral side chains have been prepared by synthetic methods suitable to achieve regioregular head-to-tail and head-to-head/tail-to-tail derivatives. In particular, the chiral (S)-(2-methyl)butyl moiety was linked at position 3 of the thiophene ring through heteroatoms
Paolo Bollella et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 21(1), 120-128 (2019-08-14)
Biocatalytic buckypaper electrodes modified with pyrroloquinoline quinone (PQQ)-dependent glucose dehydrogenase and bilirubin oxidase for glucose oxidation and oxygen reduction, respectively, were prepared for their use in a biofuel cell. A small (millimeter-scale; 2×3×2 mm3 ) enzyme-based biofuel cell was tested in
Spectroscopic studies of bipolarons from oligomerized 3-methoxythiophene in solution.
Chang A-C and Miller LL.
Synthetic Metals, 22(1), 71-78 (1987)
Blake et al.
Acta crystallographica. Section B, Structural science, 55(Pt 6), 963-974 (2000-08-06)
The intramolecular and intermolecular geometries of six thiophenes carrying oxygen-containing substituents have been determined. Crystals of 2-methoxythiophene and 3-methoxythiophene were grown in situ on a diffractometer from liquid samples. The 2-methoxy group introduces significant distortions to the thiophene nucleus and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service