Sigma-Aldrich does not test the Product W4394, Withaferin A, for solution stability. Information in our files indicates that although it is best to use freshly prepared solution when possible. However, if stock solutions must be stored, we would recommend they be stored in frozen aliquots at -20°C or below for about one month.
W4394
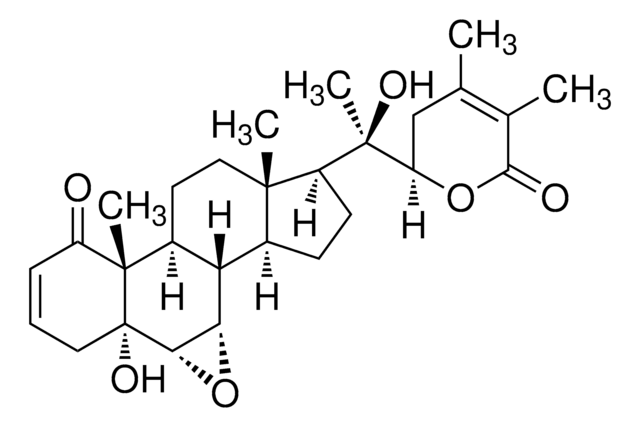
Withaferin A
≥95% (HPLC)
Synonym(s):
5,6-Epoxy-4,27-dihydroxy-1-oxowitha-2,24-dienolide, Withaferine A
About This Item
Recommended Products
biological source
plant (Withania somnifera)
Quality Level
Assay
≥95% (HPLC)
form
powder
mp
252-253 °C
functional group
epoxy
ketone
shipped in
ambient
storage temp.
2-8°C
SMILES string
C[C@H]([C@H]1CC(C)=C(CO)C(=O)O1)[C@H]2CC[C@H]3[C@@H]4C[C@H]5O[C@]56[C@@H](O)C=CC(=O)[C@]6(C)[C@H]4CC[C@]23C
InChI
1S/C28H38O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h7-8,15-16,18-21,23-24,29,31H,5-6,9-13H2,1-4H3/t15-,16-,18+,19-,20-,21+,23-,24+,26+,27-,28+/m0/s1
InChI key
DBRXOUCRJQVYJQ-CKNDUULBSA-N
Application
Biochem/physiol Actions
Preparation Note
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
438.8 °F
Flash Point(C)
226 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
What is the solution stability of Product W4394, Withaferin A?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
How does the storage temperature relate to shipping conditions?
1 answer-
The storage conditions that a Sigma-Aldrich catalog and label recommend for products are deliberately conservative. For many products, long-term storage at low temperatures will increase the time during which they are expected to remain in specification and therefore are labeled accordingly. Where short-term storage, shipping time frame, or exposure to conditions other than those recommended for long-term storage will not affect product quality, Sigma-Aldrich will ship at ambient temperature. The products sensitive to short-term exposure to conditions other than their recommended long-term storage are shipped on wet or dry ice. Ambient temperature shipping helps to control shipping costs for our customers. At any time, our customers can request wet- or dry-ice shipment, but the special handling is at customer expense if our product history indicates that the product is stable for regular shipment.
Helpful?
-
-
How does one solubilize Product W4394, Withaferin A?
1 answer-
Sigma-Aldrich tests Product W4394, Withaferin A, for solubility using methanol at a concentration of 1 mg/mL. Information in our files indicates that it is also soluble in DMSO and ethanol.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service