171778
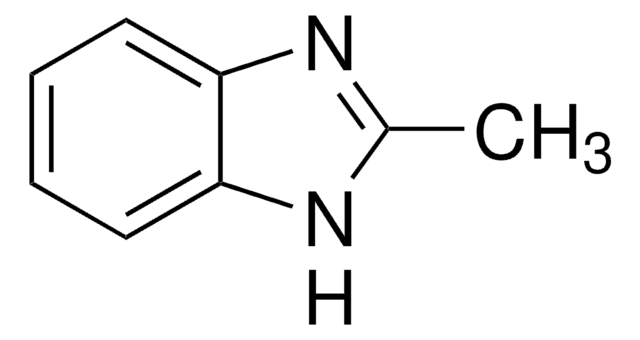
2-Aminobenzimidazole
97%
Synonym(s):
2-Benzimidazolamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H7N3
CAS Number:
Molecular Weight:
133.15
Beilstein:
116525
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
226-230 °C (lit.)
SMILES string
Nc1nc2ccccc2[nH]1
InChI
1S/C7H7N3/c8-7-9-5-3-1-2-4-6(5)10-7/h1-4H,(H3,8,9,10)
InChI key
JWYUFVNJZUSCSM-UHFFFAOYSA-N
Gene Information
human ... PLAU(5328)
Looking for similar products? Visit Product Comparison Guide
Application
2-Aminobenzimidazole was used in the hydrolysis of a choline carbonate. It was also used in the synthesis of imidazo[1,2-a]benzimidazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bartolomé Soberats et al.
Organic letters, 16(3), 840-843 (2014-01-15)
The hydrolysis of a choline carbonate through a metal-free, enzyme-like mechanism has been achieved using a 2-aminobenzimidazole-based deep cavitand as catalyst. The supramolecular catalysis involves three steps: host-guest binding, carbamoylation and enzyme-like hydrolysis. Interestingly the rate-determining step proceeds through a
Ya-Shan Hsiao et al.
ACS combinatorial science, 15(10), 551-555 (2013-09-11)
A one-pot, two-step synthesis of imidazo[1,2-a]benzimidazoles has been achieved by a three-component reaction of 2-aminobenzimidazoles with an aromatic aldehyde and an isocyanide. The reaction involving condensation of 2-aminobenzimidazole with an aldehyde is run under microwave activation to generate an imine
E Molnár et al.
Die Pharmazie, 66(9), 662-665 (2011-10-27)
Cellular drug target identification through affinity chromatography is often hindered by the quantity of nonspecific binders, such as cytoskeletal and heat shock proteins. Thus, we prepared a 2-aminobenzimidazole-tethered depletion resin designed for removal of these proteins, and tested it on
Xiao S Liu et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 44(6), 591-597 (2010-02-26)
A method using liquid chromatography and a single mix-mode solid-phase extraction cleanup for the simultaneous analysis of thiabendazole [2-(1,3-thiazol-4-yl)-1H-benzoimidazole], carbendazim [(methyl N-(1H-benzoimidazol-2-yl)-carbamate)] and 2-aminobenzimidazole (1H-benzimidazol-2-amine) in concentrated fruit juices is described. The three fungicides were isolated from the samples and
Fei Wang et al.
The Journal of organic chemistry, 76(9), 3174-3180 (2011-03-19)
An efficient strategy for the synthesis of a variety of 2-animobenzimidazole derivatives has been developed. The reaction proceeded from o-haloanilines and carbodiimides via copper(I)-catalyzed domino reaction in the presence of tert-butoxide to afford the corresponding 2-animobenzimidazole derivatives in good to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service