89064
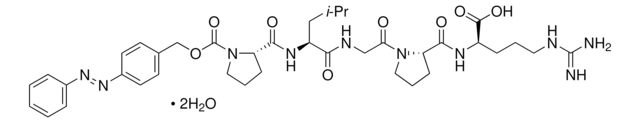
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt
collagenase substrate, chromogenic, ≥95% (HPLC), powder
Synonym(s):
Pz-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C38H52N10O8 · xC2HF3O2
Molecular Weight:
776.88 (free base basis)
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
product name
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt, ≥95% (HPLC)
Assay
≥95% (HPLC)
form
powder
Application
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt has been used as substrate for collagenase.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly sensitive assay for PZ-peptidase activity by high-performance liquid chromatography
Chikuma, T., et al.
Journal of Chromatography A, 348, 205-212 (1985)
T Chikuma et al.
Journal of chromatography, 635(1), 81-87 (1993-04-09)
A rapid and sensitive assay method for the determination of PZ-peptidase activity is reported. This method is based on the monitoring of the absorption at 320 nm of 4-phenylazobenzyloxycarbonyl-L-Pro-L-Leu (PZ-Pro-Leu), enzymatically formed from the substrate 4-phenylazobenzyloxycarbonyl-L-Pro-L-Leu-Gly-L-Pro-D-Arg (PZ-peptide), after separation by
U Tisljar
Biological chemistry Hoppe-Seyler, 374(2), 91-100 (1993-02-01)
Thimet oligopeptidase (EC 3.4.24.15) is a thiol-dependent metallo-endopeptidase also known as Pz-peptidase, collagenase-like peptidase, endooligopeptidase A, soluble metallo-endopeptidase and endopeptidase 24.15. The enzyme is closely related to the yeast proteinase yscD. Thimet oligopeptidase (M(r) 74000) is widely distributed in animals
A Study of the Collagen-binding Domain of a 116-kDaClostridium histolyticum Collagenase*
Osamu Matsushita
The Journal of Biological Chemistry (1998)
The induction of collagenase and a neutral proteinase by their high molecular weight substrates in Achromobacter iophagus.
V Keil-Dlouha et al.
Journal of molecular biology, 107(3), 293-305 (1976-11-05)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala](/deepweb/assets/sigmaaldrich/product/structures/805/876/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85/640/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85.png)