930687
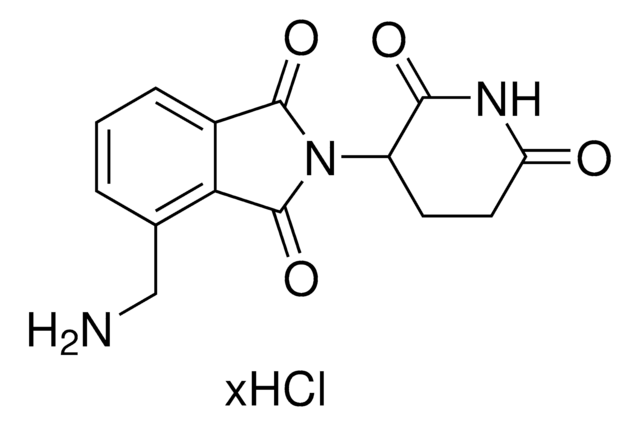
2,6-Piperidinedione, 3-[(3-aminophenyl)amino] hydrochloride
≥95%
Synonym(s):
3-((3-Aminophenyl)amino)piperidine-2,6-dione hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H13N3O2 · xHCl
CAS Number:
Molecular Weight:
219.24 (free base basis)
UNSPSC Code:
12352200
NACRES:
NA.28
Recommended Products
Quality Level
Assay
≥95%
reaction suitability
reagent type: ligand
functional group
amine
storage temp.
2-8°C
SMILES string
O=C1NC(C(CC1)NC2=CC(N)=CC=C2)=O.[xHCl]
Application
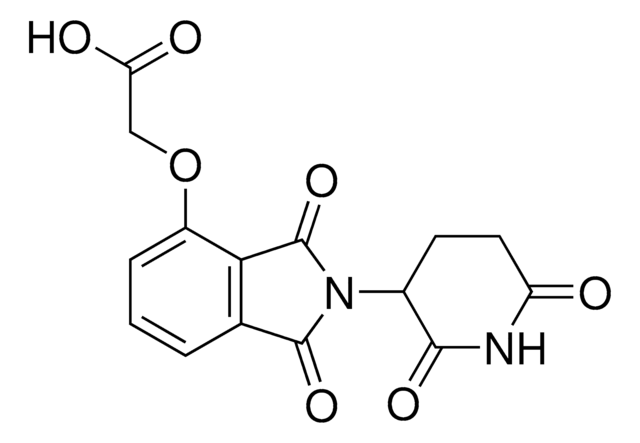
2,6-Piperidinedione, 3-[(3-aminophenyl)amino] hydrochloride is a functionalized Cereblon ligand used for development of protein degrader building blocks. Contains a terminal amine group, allowing rapid conjugation of carboxyl containing linkers. A basic building block for development of a protein degrader library.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Targeted Protein Degradation by Small Molecules
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jingwei Shao et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 8(20), e2102555-e2102555 (2021-08-17)
DNA-binding proteins, including transcription factors (TFs), play essential roles in various cellular processes and pathogenesis of diseases, deeming to be potential therapeutic targets. However, these proteins are generally considered undruggable as they lack an enzymatic catalytic site or a ligand-binding
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service