H36605
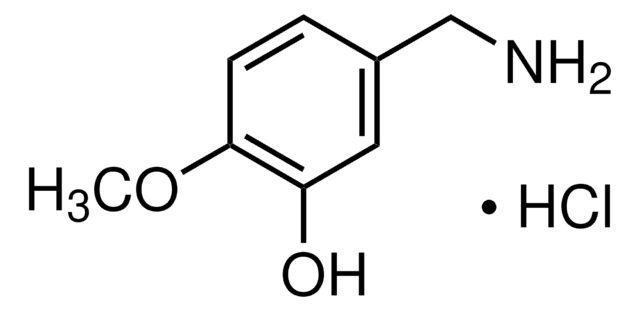
4-Hydroxy-3-methoxybenzylamine hydrochloride
98%
Synonym(s):
Vanillylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CH2NH2 · HCl
CAS Number:
Molecular Weight:
189.64
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
219-221 °C (dec.) (lit.)
SMILES string
Cl.COc1cc(CN)ccc1O
InChI
1S/C8H11NO2.ClH/c1-11-8-4-6(5-9)2-3-7(8)10;/h2-4,10H,5,9H2,1H3;1H
InChI key
PUDMGOSXPCMUJZ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suvi F Flagan et al.
Environmental microbiology, 8(3), 560-565 (2006-02-16)
Capsaicin contributes to the organoleptic attributes of hot peppers. Here, we show that capsaicin is utilized as a growth nutrient by certain bacteria. Enrichment cultures utilizing capsaicin were successfully initiated using Capsicum-derived plant material or leaves of tomato (a related
Bellur Chayapathy Narasimha Prasad et al.
Journal of agricultural and food chemistry, 54(18), 6660-6666 (2006-08-31)
Capsaicin, a pungency factor alkaloid of Capsicum fruits, is biosynthesized by enzymatic condensation of vanillylamine, a phenyl propanoid intermediate, and 8-methyl-nonenoic acid, a fatty acid derivative from the leucine/valine pathway by capsaicin synthase. Biotic elicitors, such as aqueous mycelial extracts
Harishchandra B Gururaj et al.
Plant science : an international journal of experimental plant biology, 195, 96-105 (2012-08-28)
Capsaicinoid biosynthesis involves the participation of two substrates viz. vanillylamine and C(9)-C(11) fatty acid moieties. Vanillylamine which is a derivative of vanillin is synthesized through a transaminase reaction in the phenylpropanoid pathway of capsaicinoid synthesis. Here we report the functional
Kenji Kobata et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1611-1614 (2011-08-09)
Stable isotope-labeled precursors were synthesized for an analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to elucidate the biosynthetic flow of capsaicinoids, capsinoids, and capsiconinoids. [1'-(13)C][5-(2)H]-Vanillin was prepared by the condensation of guaiacol with [(13)C]-chloroform and a D(2)O treatment. Labeled vanillylamine
Yaqin Lang et al.
The Plant journal : for cell and molecular biology, 59(6), 953-961 (2009-05-29)
Capsaicinoids are responsible for the spicy flavor of pungent peppers (Capsicum). The cultivar CH-19 Sweet is a non-pungent pepper mutant derived from a pungent pepper strain, Capsicum annuum CH-19. CH-19 Sweet biosynthesizes capsaicinoid analogs, capsinoids. We determined the genetic and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service