O003
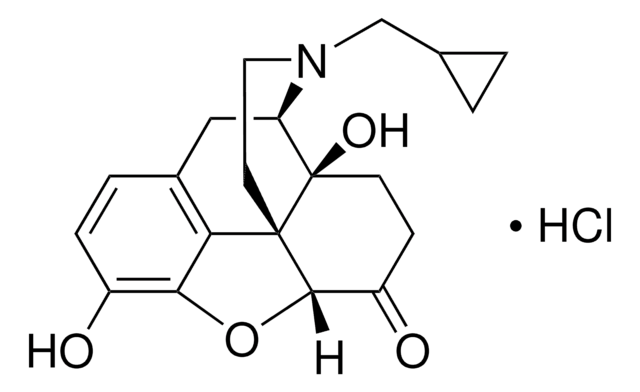
β-Funaltrexamine hydrochloride
solid
Synonym(s):
β-FNA hydrochloride, (E)-4-[[(5α,6β)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-yl]amino]-4-oxo-2-butenoic acid methyl ester hydrochloride
About This Item
Recommended Products
form
solid
drug control
regulated under CDSA - not available from Sigma-Aldrich Canada
storage condition
desiccated
color
white
solubility
H2O: 7.5 mg/mL (aqueous solutions should be promptly used)
methanol: 7.6 mg/mL (do not store in ethanolic solution; methanolic solutions may be stored for several weeks at 4 °C)
storage temp.
−20°C
SMILES string
Cl.COC(=O)\C=C\C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5OC1[C@]2(CCN3CC6CC6)c45
InChI
1S/C25H30N2O6.ClH/c1-32-20(30)7-6-19(29)26-16-8-9-25(31)18-12-15-4-5-17(28)22-21(15)24(25,23(16)33-22)10-11-27(18)13-14-2-3-14;/h4-7,14,16,18,23,28,31H,2-3,8-13H2,1H3,(H,26,29);1H/b7-6+;/t16-,18-,23+,24+,25-;/m1./s1
InChI key
BIPHUOBUKMPSQR-NQGXHZAGSA-N
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
LC/MS Analysis of Opioid Glucuronide Metabolites in Urine on Ascentis® Express F5 after Solid Phase Extraction (SPE) using Supel™-Select HLB
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service