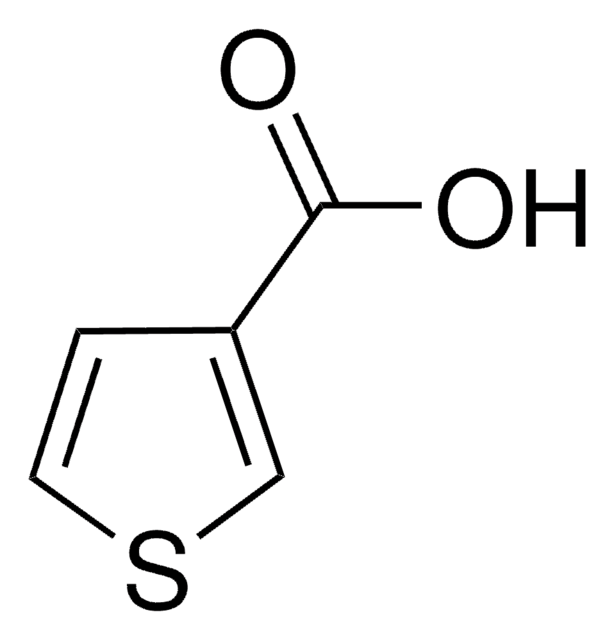
F20505
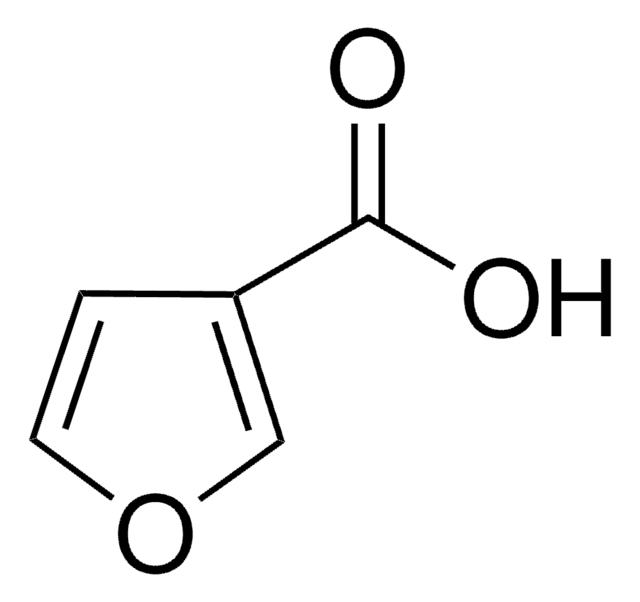
2-Furoic acid
98%
Synonym(s):
Furan-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H4O3
CAS Number:
Molecular Weight:
112.08
Beilstein:
110149
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
230-232 °C (lit.)
mp
128-132 °C (lit.)
SMILES string
OC(=O)c1ccco1
InChI
1S/C5H4O3/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
InChI key
SMNDYUVBFMFKNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Some of the applications of 2-furoic acid include:
- Synthesis of a single-molecule magnet, Mn11Gd2 based high-nuclearity heterometallic complex.
- Synthesis of orally active antidiabetic vanadyl complex, bis(α-furancarboxylato)oxovanadium(IV).
- As a ligand in the synthesis of luminescent 4f-3d heterometallic one-dimensional coordination polymers.
- Synthesis of biocompatible multifunctional dextran furoate nanospheres.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
282.7 °F - closed cup
Flash Point(C)
139.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Syntheses, crystal structure and luminescence property of novel 4f-3d heterometallic one-dimensional coordination polymers.
Yin M.
Journal of Physics and Chemistry of Solids, 67(7), 1372-1378 (2006)
Structure Design of Multifunctional Furoate and Pyroglutamate Esters of Dextran by Polymer?Analogous Reactions.
Hornig S.
Macromolecular Bioscience, 7(3), 297-306 (2007)
Jin-Xin Gao et al.
Phytopathology, 104(4), 332-339 (2013-10-19)
The maize pathotype Cochliobolus lunatus causes Curvularia leaf spot by producing a non-host-specific toxin known as methyl 5-(hydroxymethyl) furan-2-carboxylate (M5HF2C). However, related research that explores the genes that control the production of this toxin is rare. In the current work
A new orally active antidiabetic vanadyl complex-bis (α-furancarboxylato) oxovanadium (IV).
Xie M.
Journal of Inorganic Biochemistry, 99(2), 546-551 (2005)
A bell-shaped Mn11Gd2 single-molecule magnet.
Mereacre VM.
Journal of the American Chemical Society, 129(30), 9248-9249 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service