CDS023093
Nilotinib
Synonym(s):
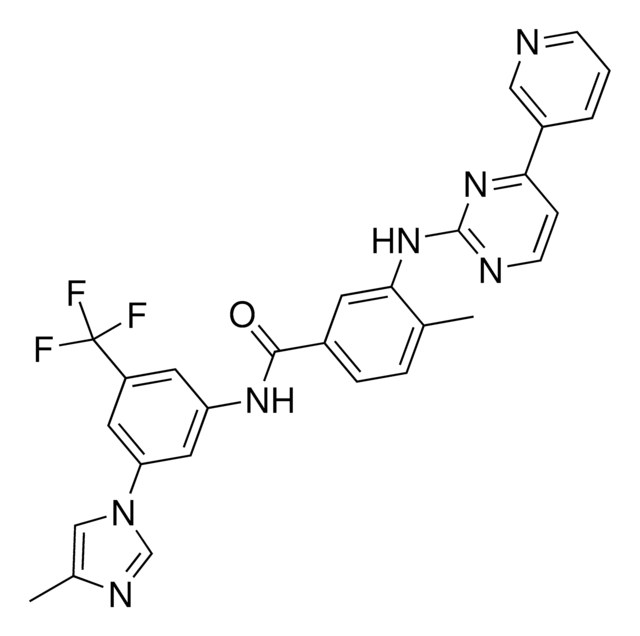
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-N-[5-(4-methyl-1H-imidazol-1-yl)-3-(trifluoromethyl)phenyl]benzamide, 4-Methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzamide, 4-Methyl-N-[3-(4-methylimidazol-1-yl)-5-trifluoromethylphenyl]-3-[[4-(pyridin-3-yl)pyrimidin-2-yl]amino]benzamide
About This Item
Recommended Products
description
AldrichCPR
form
solid
storage temp.
-10 to -25°C
SMILES string
CC1=CN(C2=CC(NC(C3=CC=C(C(NC4=NC=CC(C5=CC=CN=C5)=N4)=C3)C)=O)=CC(C(F)(F)F)=C2)C=N1
InChI
1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37)
InChI key
HHZIURLSWUIHRB-UHFFFAOYSA-N
Gene Information
human ... ABL1(25)
General description
Other Notes
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY, (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY, WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - STOT RE 1
Target Organs
Liver,Kidney,gallbladder
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service