A2765
Azocasein
protease substrate, chromogenic, powder
Synonym(s):
Sulfanilamide-azocasein
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
product name
Azocasein, protease substrate
biological source
bovine
form
powder
technique(s)
ligand binding assay: suitable
solubility
water: 5 mg/mL, clear, orange to very deep orange
ε (extinction coefficient)
≥25 at 440 nm in 0.1 M NaOH at 1%
storage temp.
2-8°C
General description
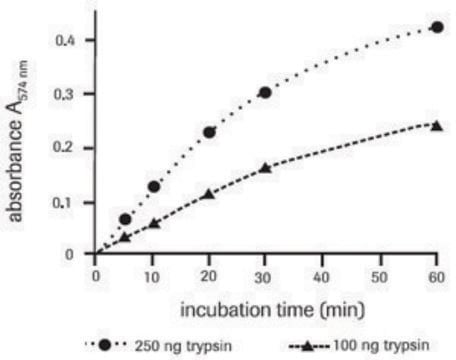
Azocasein is a chromogenic derivative of casein. Protease degrades azocasein to yield TCA-soluble azopeptides with high UV-absorbance. This azocasein assay is widely employed to estimate the protease production by bacterial fermentation on synthetic substrates from glucose and inorganic salts.
Application
Azocasein has been used as a substrate for determination of protease activity.
Azocasein is a nonspecific protease substrate. Hydrolysis of the casein releases the azo dye into the media where it is detected by absorbance at 440 nm.
Azocasein is an inflammatory agent that is used to induce amyloid A amylooidosis in experimental animals.
Linkage
View more information on azocasein protease assay at www.sigma-aldrich.com/enzymeexplorer
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Isolation and characterization of chitinolytic Streptomyces sp. MT7 and its antagonism towards wood-rotting fungi
Nagpure, A, et al.
Annals of Microbiology, 64(2), 531-541 (2014)
Azocasein assay for alkaline protease in complex fermentation broth
Iversen SL and Jorgensen MH
Biotechnol. Tech., 9(8), 573-576 (1995)
Zhisheng Xu et al.
PloS one, 6(8), e23562-e23562 (2011-09-03)
Halolysin SptA from haloarchaeon Natrinema sp. J7 consists of a subtilisin-like catalytic domain and a C-terminal extension (CTE) containing two cysteine residues. In this report, we have investigated the function of the CTE using recombinant enzymes expressed in Haloferax volcanii
Prevalence and characterization of Bacillus cereus group from various marketed dairy products in India
Kumari S and Sarkar PK
Dairy science & technology, 94(5), 483-497 (2014)
Recombinant proteins from plants: production and isolation of clinically useful compounds, 3 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service