All Photos(1)
About This Item
Empirical Formula (Hill Notation):
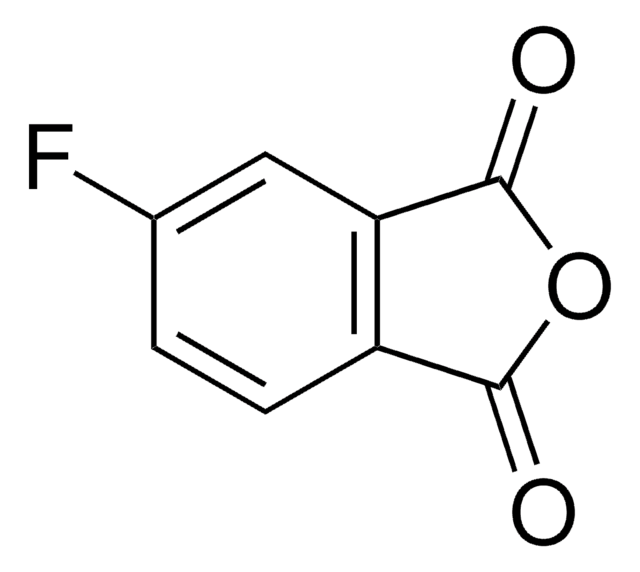
C8H3FO3
CAS Number:
Molecular Weight:
166.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
158-161 °C (lit.)
SMILES string
Fc1cccc2C(=O)OC(=O)c12
InChI
1S/C8H3FO3/c9-5-3-1-2-4-6(5)8(11)12-7(4)10/h1-3H
InChI key
WWJAZKZLSDRAIV-UHFFFAOYSA-N
General description
3-Fluorophthalic anhydride undergoes reduction with NaBH4 to afford 4-fluorophthalide and 7-fluorophthalide. It can be synthesized starting from 3-nitrophthaloyl dichloride.
Application
3-Fluorophthalic anhydride may be used in the preparation of substituted benzamides with potential neuroleptic activity. It may be employed as starting reagent for the synthesis of 8-fluoro-10-methyl-1,2-benzanthracene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Notes-Preparation of 3-Fluorophthalic Anhydride.
Heller A.
The Journal of Organic Chemistry, 25(5), 834-835 (1960)
Regioselectivity of metal hydride reductions of unsymmetrically substituted cyclic anhydrides. systems where" steric hindrance along the preferred reaction path" rationalization is not applicable.
Kayser MM and Morand P.
Canadian Journal of Chemistry, 58(23), 2484-2490 (1980)
Synthesis of 8-Fluoro-10-methyl-1, 2-benzanthracene1.
Newman MS and Wiseman EH.
The Journal of Organic Chemistry, 26(9), 3208-3211 (1961)
Yingming Wang et al.
Colloids and surfaces. B, Biointerfaces, 188, 110795-110795 (2020-01-29)
Anaplastic lymphoma kinase (ALK) is a major target in treating non-small-cell lung cancer, and several ALK inhibitors have been developed to antagonize its kinase activity. However, patients treated with inhibitors ultimately develop drug resistance. Therefore, therapies with new mechanisms of
A new synthesis of 3-fluorophthalic anhydride.
Passudetti M, et al.
Journal of Fluorine Chemistry, 50(2), 251-255 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service