A15207
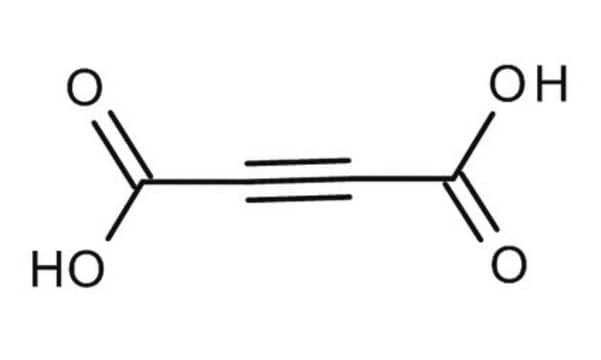
Acetylenedicarboxylic acid
95%
Synonym(s):
2-Butynedioic acid
About This Item
Recommended Products
Assay
95%
form
crystals
mp
180-187 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)C#CC(O)=O
InChI
1S/C4H2O4/c5-3(6)1-2-4(7)8/h(H,5,6)(H,7,8)
InChI key
YTIVTFGABIZHHX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Some of the common applications are listed below:
- Acetylenedicarboxylic acid and its ester derivatives can undergo Cu(I)-catalyzed radical addition with acetophenones to yield furan derivatives.
- It is one of the shortest linear links used in the preparation of Covalent Organic Frameworks (COFs) and Metal Organic Frameworks (MOFs) like IRMOF-0.
- Various hexaarylbenzenes can be built via oxidative cyclodehydrogenation of diaryl alkynes derived from acetylenedicarboxylic acid.
- It can be used as a spacer while synthesizing molecular chains or dendrimers such as 2,3,8-trifunctionalized indenoquinoxaline dendrons and bisarylacetylene chromophores.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
267.8 °F
Flash Point(C)
131 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service