All Photos(1)
About This Item
Empirical Formula (Hill Notation):
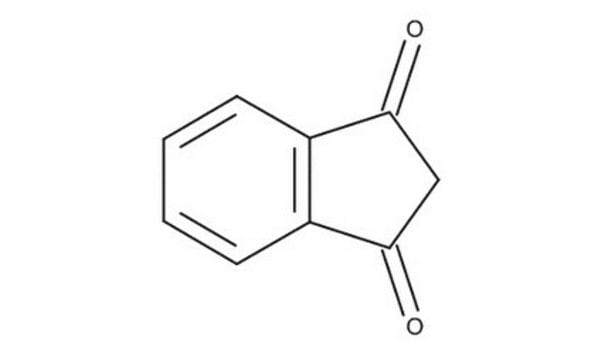
C9H6O2
CAS Number:
Molecular Weight:
146.14
Beilstein:
1210061
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
129-132 °C (lit.)
SMILES string
O=C1CC(=O)c2ccccc12
InChI
1S/C9H6O2/c10-8-5-9(11)7-4-2-1-3-6(7)8/h1-4H,5H2
InChI key
UHKAJLSKXBADFT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bilquees Bano et al.
Bioorganic chemistry, 81, 658-671 (2018-09-27)
Current study deals with the evaluation of indane-1,3-dione based compounds as new class of urease inhibitors. For that purpose, benzylidine indane-1,3-diones (1-30) were synthesized and fully characterized by different spectroscopic techniques including EI-MS, HREI-MS, 1H, and 13C NMR. All synthetic
[Effect of S-oxidation on the anticoagulant effects of 4-hydroxycoumarins, 4-hydroxy-2-pyrones and 1,3-indanediones].
K Rehse et al.
Archiv der Pharmazie, 317(3), 262-267 (1984-03-01)
Majid M Heravi et al.
Molecular diversity, 13(3), 385-387 (2009-03-03)
Aldehydes, 1,3-indandione and cyclohexylisocyanide undergo smooth coupling-cyclization in water to produce the corresponding 2-(cyclohexylamino)-3-aryl- indeno [1,2-b] furan-4-ones in good yields. Water was used as a solvent to avoid the use of other highly toxic and environmentally unfavorable solvents for this
Abdolmajid Bayandori Moghaddam et al.
Chemical & pharmaceutical bulletin, 54(10), 1391-1396 (2006-10-04)
This is an environmentally friendly method in the field of electroorganic reactions under controlled potential electrolysis, without toxic reagents at a carbon electrode in an undivided cell which involves the (EC) mechanism reaction and comprises two steps alternatively; (i) electrochemical
A R Murthy et al.
Journal of medicinal chemistry, 28(11), 1591-1596 (1985-11-01)
A series of 2-substituted indan-1,3-dione derivatives, including alkyl (C-1-C-5), mono- and disubstituted phenyl, and other 2-aryl derivatives, were tested for hypolipidemic activity of CF1 male mice at 20 mg/kg per day. These derivatives reduced both serum cholesterol and triglycerides after
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service