N-Heterocyclic Carbenes
Enyne Cyclizations
Echavarren and co-workers have also demonstrated the utility of Au-NHCs in the reactions of 1,6-enynes. They determined that the outcome of the cyclization of 1,6-enyne 1 (Scheme 1) was dependent on the ligand coordinated to the metal. When 2 was used in combination with AgSbF6, the major product resulted from 5-exodig cyclization to afford a cyclopropyl metal carbene species, which upon reaction with the nucleophile afforded the carbocyclic product (see previous section). On the other hand, when the carbene-based Au-complex was used, the cyclopropyl derivative was observed as the major product.1

Scheme 1.
Rearrangement of Allylic Acetates
Marion and co-workers reported the first rearrangement of allylic acetates using a gold catalyst ligated to an N-heterocyclic carbene ligand. A bulky ligand bound to the gold catalyst proved imperative to this approach. The catalyst system was found to be highly versatile, providing a variety of rearranged primary oxo derivatives in good yield, either under thermal or microwave conditions (Scheme 2). In addition, the scope was found to be quite broad for a wide variety of allylic acetates. The authors propose a mechanism akin to that described by Overman, Henry, and Hartwig for analogous allylic rearrangments with Hg and Pd (cyclization-induced rearrangement-Scheme 3).2

Scheme 2.

Scheme 3.
N-Heterocyclic Carbene-Copper Complexes
N-heterocyclic carbene ligands have proven very popular in the last 20 years. The electronic and steric modularity associated with the resulting complexes have made NHCs obvious candidates when designing new metal complexes for catalysis. Nolan and co-workers are among the pioneers in the use of NHC ligands for catalysis and they have reported the use of Cu-NHCs for a variety of catalytic transformations. Conjugate reduction of a,ß-unsaturated ketones and esters, the hydrosilylation of ketones, the cyclopropanation of terminal alkenes, as well as olefinations, carbene transfer reactions, aziridination of olefins, and methylenation of aldehydes (Scheme 4) are among some examples of the uses of Cu-NHC complexes (specifically (IPr)CuCl) in modern catalysis. Finally, these catalysts are air- and moisture-stable, and they can be used as precursors to synthesize more air-sensitive complexes.3

Scheme 4.
Nolan and co-workers have described the use of the Cu-Imes carbene complex 5 in the olefination of aldehydes (and ketones) in the presence of PPh3, i-PrOH, and TMSCHN2. While similar olefinations have been reported using Wilkinson’s catalyst, the Cu-alternative offers a more economical solution. The functional group compatibility is quite good, allowing for the formation of functionalized aliphatic olefins, dienes, and styrenes containing nitro (notoriously deleterious to these types of reactions), trifluoromethyl, amino, and ester functionalities as well as for heteroaromatic olefins substituted with pyridine, pyrrole, and indole derivatives (Scheme 5). The isopropyl-derived carbene complex 6 was also demonstrated to be quite useful in these types of reactions under slightly modified reaction conditions (Scheme 6).4

Scheme 5.
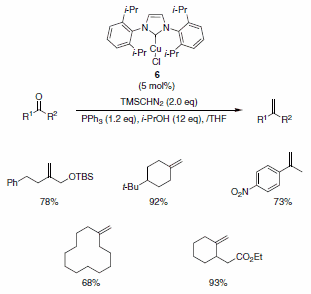
Scheme 6.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?